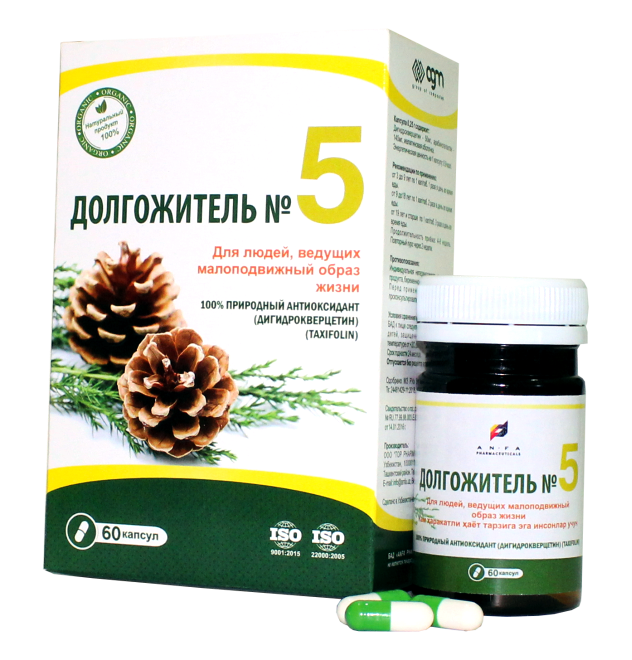
DOLGOJITEL №5
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL №5 – FOR PEOPLE WITH PHYSICAL DISABILITIES
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 5, 0.25 gr. in each capsules, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 92% | 50 mg. |
| Arabinogalactan | 140 mg. |
| Starch | 60 mg. |
EFFECT OF THE MEDICINE
- The preparation is intended for people with physical disabilities and for those who have to lead a sedentary lifestyle.
- Dihydroquercetin and arabinogalactan help to ensure the normal functioning of the body, deprived of the natural possibilities.
- This complex increases vitality, thereby improving the quality of life and increasing its endurance.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
As a means of prevention:
During immunity disorders:
- recommended as a tonic to enhance the body’s defenses;
- increases the body’s resistance to infectious diseases and the harmful effects of the environment.
During exacerbation and complication of inflammatory diseases:
- reduces the severity of inflammatory processes;
- staves off exacerbations and recurrence of chronic inflammatory diseases.
In cardiovascular diseases:
- contributes to the normalization of blood pressure;
- improves blood microcirculation and cerebral circulation;
- reduces the effects of hypoxia and ischemia.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №3
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL № 3 – FOR CARDIOVASCULAR SYSTEM
COMPOSITION AND DOSAGE FORM
DOLGOJITEL №3, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with a protective holographic label; (2) – instruction for use;
Composition:
| Dihydroquercetin (Taxifolin) 95% | 50 mg. |
| Acidum ascorbinicum | 125 mg. |
| Starch | 75 mg. |
EFFECT OF THE MEDICINE
- strengthens the walls of blood vessels and capillaries, increasing their strength and elasticity;
- increases blood flow velocity, while improving microcirculation and the tone of the capillary bed;
- prevents the formation of internal blood clots, which is the cause of many cardiovascular diseases;
- improves the rheological properties of blood, its viscosity, and clotting, weakens the aggregation of red blood cells, improves their deformability in diseases involving increased blood viscosity syndrome (cerebral ischemia of the brain and heart, hypertension, diabetes);
- evinces membrane stabilizing activity, reducing the severity of the inflammatory process;
- reduces the level of cholesterol, lipids in the liver and serum;
- inhibits age-related changes in blood vessels, while slowing the progression of vascular diseases;
- reduces blood clots and the risk of progression of diabetic vascular complications, positively affecting the permeability of blood vessels.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
In the complex therapy of cardiovascular diseases:
In atherosclerosis:
- During the course of treatment with Dolgojitel №3, the total cholesterol concentration decreases, as well as the coefficient of atherogenic serum decreases, and triglyceride levels in the blood reduces. This helps to reduce the clinical presentations of atherosclerosis.
In ischemic heart disease, heart failure, heart rhythm disorders:
- Dolgojitel №3 helps to improve the rheological properties of blood, improves the condition of the membranes, increases their deformability, improves hemodynamic parameters.
During arterial hypertension of I and II degree.
- Under the influence of Dolgojitel №3, blood pressure normalizes, the amplitude of its daily fluctuations decreases, vascular tone ameliorates, cerebral circulation improves. As a result, it decreases headache and dizziness.
For prevention and in the period of rehabilitation after a stroke and heart attack.
- Dolgojitel №3 restores microcirculation, helps to improve venous outflow, reduces hypoxia, renders a positive effect on metabolic processes in cells. Moreover, it helps to prevent the recurrence of the disease.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №2
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
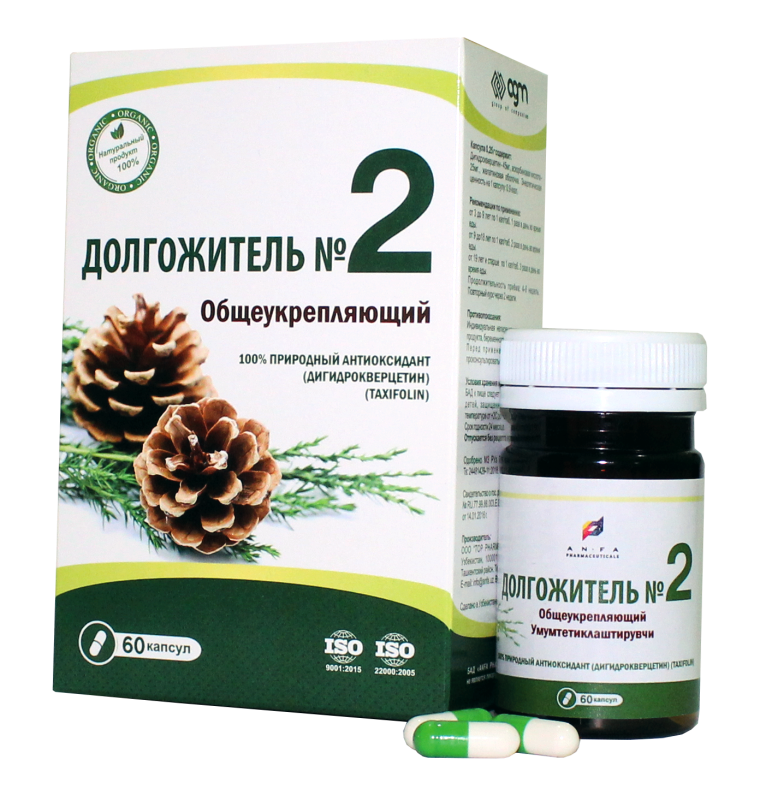
DOLGOJITEL №2 – RESTORATIVE
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 2, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 92% | 45 mg. |
| Acidum ascorbinicum | 25 mg. |
| Starch | 180 mg. |
INDICATIONS
- Restorative agent, indispensable for any person in a modern rhythm of life;
- General languor, a decrease in physical and intellectual performance, absent-mindedness, depression – these are signs of inadequate tissue supply with nutrients and oxygen as well as an insufficient rate of excretion of degraded products.
- Dihydroquercetin and its derivatives, through strengthening tissues of blood vessels, contribute to the restoration of their transport function and the disposal of “manager’s syndrome”;
- Taxifolin is an excellent preventive agent for slowing down the aging process in the body.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
In case of immunity disorders:
- It is recommended as a tonic to strengthen the body’s defenses, resistance to infectious diseases and the harmful effects of the environment.
In exacerbations and complications of inflammatory diseases:
- resistance improves, the severity of the inflammatory process reduces, the exacerbation and recurrence of chronic inflammatory diseases stave off.
In the course of liver dysfunction when it is impaired:
- the functional condition of the liver cells improves, and its antitoxic function will be maintained.
In varicose veins, phlebothrombosis, with post-thrombophlebitic syndrome:
- strengthens the venous walls, improves venous blood flow, prevents thrombosis and reduces edema.
In the complications of diabetes:
- improves microcirculation, strengthens the capillary bed, reduces insulin resistance, and improves the quality of life.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label,
(2) – instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic, diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Biologic corrector of carbohydrate metabolism;
- Normalizes carbohydrate metabolism and lowers blood sugar levels;
- Compensates energy deficit;
- When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
- In Obesity, medicine contributes to the reduction and stabilization of weight;
- Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
INSIDE
2 tablets 3 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
GINKO CARDIOZEN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Ginkgo biloba (Ginkgo L.)
Elecampane (Inula helenium L.)
Midland hawthorn (Crataegus laevigata)
Valerian herb (Valeriana officinalis)
Lemon balm (Melissa officinalis)
Common yarrow (Achillea millefolium L)
Motherwort (Leonúrus)
Peppermint (Mentha piperita L.)
effect of the medicine
- GINKO CARDIOZEN – a preparation of herbal origin. The medicine has a spasmolytic effect of myotropic nature (similar to the effect of papaverine), mainly in relation to coronary vessels.
- 3-5 days after the intake of Ginko Cardiozen, the therapeutic effect of the medicine occurs – attenuation is observed or elimination of the manifestations of diseases (elimination or significant attenuation of chest pains, pain in the heart, etc.).
- Treatment with Ginko Cardiozen significantly reduces and weakens the attacks of angina, patients less often resort to the use of nitroglycerin, Validol, and other vasodilators.
- Contributes to the normalization of the heart rate. The medicine contributes to the normalization of the contractile function of the myocardium, improving conductivity, and provides nutrition to the heart muscle.
- The main components of the medicine (hawthorn and motherwort) affect all the basic mechanisms that optimize the work of the heart, improves the nutrition of the heart muscle, by dilating the coronary vessels, improves metabolic processes in the myocardium at the cellular level, regulates the heart rate.
- Moreover, possesses a sedative effect, which is important in the prevention of heart diseases.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine GINKO CARDIOZEN is prescribed for:
- heart failure;
- cardioneurosis;
- cardio-psychoneurosis;
- arterial hypertension;
- cardiac ischemia;
- chronic coronary insufficiency; (manifested by pain behind the sternum and in the region of the heart at rest or after physical exertion).
- myocardial dystrophy of different genesis;
- disorders of cerebral circulation;
CONTRAINDICATIONS
- diagnosed gastritis of erosive type;
- stomach ulcer;
- peptic ulcer of the duodenum in the stage of exacerbation;
- acute myocardial infarction;
- stable low blood pressure – (Arterial hypotension);
- disorders of cerebral circulation in an acute phase.
- hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually. GINKO CARDIOSEN is recommended to take:
Inside
1 tablet 2-3 times a day, for half an hour before meals.
The duration of treatment with the medicine (from 2 to 4 weeks) is determined by the patient’s individual response to treatment.
Repeated use of the medicine is recommended after the interval of 5-10 days. The medicine can be used not only in inpatient but also in outpatient basis.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
TROMBORON
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Ginkgo biloba (Ginkgo biloba L.)
Feverfew (Tanacetum parthenium L..)
Garlic (Allium sativum L.)
Ginger (Zingiber officinale)
Garden angelica (Angelica archangelica officinalis)
EFFECT OF THE MEDICINE
- Tromboronis designed for the purification of vessels from excess cholesterol in hyperlipidemia IIa type.
- Tromboron significantly reduces the level of total cholesterol, reduces the concentration “bad” cholesterol and improves the concentration of “good” cholesterol that is vital for the proper functioning of the brain and the production of hormones such as testosterone and estrogen.
- Tromboronis used as a prophylaxis for the protection of blood vessels of the heart and brain from cholesterol.
- It is recommended to begin prophylaxis to all above35-40 years.
- With regular admission, Tromboron removes cholesterol from the body and prevents its deposition on the walls of the vessels in the form of atherosclerotic plaques.
- Moreover, Troboron normalizes the permeability of the vascular wall.
SIDE EFFECTS
Usually, side effects do not occur, in most cases, the medication is well tolerated.
There may be allergic reactions (itching, rash), as well as nausea, headache, a feeling of bitterness in the mouth.
Use of the medicine should be discontinued when hypersensitivity reactions occur and consult a doctor.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicineTromboron is prescribed for:
- hyperlipidemia type IIa according to Frederickson, weakly expressed;
- prophylaxis of acute myocardial infarction in the presence of risk factors (such as diabetes mellitus, hyperlipidemia, arterial hypertension, obesity, smoking, old age);
- secondary prophylaxis of myocardial infarction;
- unstable angina;
- prevention of stroke (including in patients with transient disorders of cerebral circulation);
- prevention of thromboembolism after surgery and invasive vascular interventions (such as coronary artery bypass grafting, carotid endarterectomy, arteriovenous shunting, carotid angioplasty);
- prevention of deep vein thrombosis and thromboembolism of the pulmonary artery and its branches (for example, with prolonged immobilization as a result of extensive surgical intervention).
CONTRAINDICATIONS
During pregnancy and lactation.
Hypersensitivity to some components of the medicine, under the age of 18, and in cases of brain injury, brain diseases, liver disease, severe renal dysfunction, alcoholism.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually. Tromboron is recommended for:
INSIDE
2 tablets, 2-3 times a day for half an hour before meals.
The duration of treatment with the medicine (from 3 to 6 months) is determined by the patient’s individual response to treatment.
Conducting a second course of treatment is possible on the recommendation of a doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic,
diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
– Biologic corrector of carbohydrate metabolism;
– Normalizes carbohydrate metabolism and lowers blood sugar levels;
– Compensates energy deficit;
– When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
– Obesity, contributes to the reduction and stabilization of weight;
– Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
SYRUP
2.5-5.0 ml 3 times a day
The medicine is administered with meals with plenty of water.
Course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
ANEMAK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Common Nettle Extract (Urtica dioica L.)
Eleutherococcus Extract (Eleutherococcus senticosus)
Milfoil Extract (Achillea millefolium L.)
Aloe Extract (Aloe arborescens Mill.)
Trifid Bur-marigold Extract (Bidens Tripartita)
Excipients: purified water up to 100 ml, Sugar, Ethyl alcohol (95%)
EFFECT OF THE MEDICINE
– When using the medicine has a gradual regression effect on clinical and laboratory symptoms of anemia;
– Restores iron deficiency in the body and stimulates the synthesis of hemoglobin in iron deficiency anemia;
– Increases iron absorption in the intestine.
– It is a source of iron and essential amino acids;
– It helps overcome fatigue, depression, frequent tiredness, dizziness, increases the body defenses;
INDICATIONS
– Anemia (iron deficiency of different degrees of severity);
– Vitamin B12 deficiency anemia;
– Pregnancy and lactation (prevention of iron deficiency during pregnancy and lactation);
– Retardation of physical and mental development, and intensive growth;
– Sports and physical exercise, intense physical labor;
– Poor nutrition, unbalanced diet;
– Frequent acute respiratory viral infections and acute respiratory diseases (in the complex therapy);
– Donation of blood;
– For patients after various surgical measures;
– At prolonged bleeding;
– For children in adolescence and for adults (e.g., vegetarians and the elderly);
– Prevention of iron deficiency anemia in patients of a risk-group in the case where there is no possibility to ensure adequate intake of iron from food.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Anemak is recommended to take:
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
CHILDREN BETWEEN 3 AND 12 YEARS OF AGE
5 – 10 ml., 3 times a day with a meal
2,5 – 5 ml., 2-3 times a day with a meal
For peroral use, 5-10 minutes after the meal.
The course of administration is usually 30 days.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation. The use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
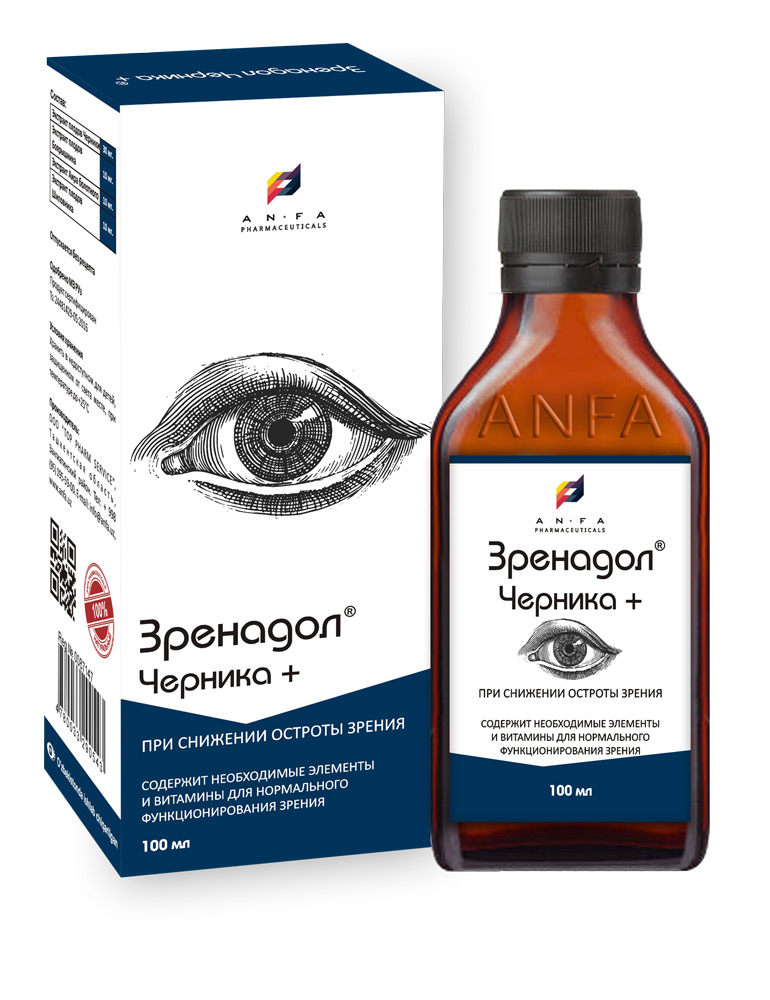
ZRENODOL CHERNIKA+
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts and their fruits, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
| Composition: | |
| Blueberry Fruit Extract (Vaccinium myrtillus) | |
| Hawthorn Fruit Extract (Crataegus) | |
| Sweet flag Extract (Acorus calamus) | |
| Rose Hip Extract (Rosa majalis) |
EFFECT OF THE MEDICINE
- ZRENADOL CHERNIKA+ protects the eyes from fatigue, irritation, and vision weakening;
- improves dark adaptation;
- reduces the permeability of capillaries, strengthens the walls of blood vessels, including the vessels of the eye fundus, reduces swelling and redness;
- supports visual organs with essential nutrients – vitamins and micronutrients;
- Tends to have a positive effect on the functional status of the visual organs associated with the deficiency of vitamins and micronutrients, has a health-promoting effect and is a natural product.
- Blueberry’s anthocyanosides contribute to the regeneration of the photosensitive pigment of the retina, increasing its sensitivity to changes in light intensity, improving visual acuity during low-light conditions; contributes to the improvement of dark adaptation.
- Active substances of Blueberry improve the flexibility of cell membranes and help to enhance blood flow to the retina.
- Blueberry extract and vitamin C (the main component of rose hips) with bioflavonoids promote the blood circulation in the vision organs, reduce intraocular pressure and have strong antioxidant properties, i.e. prevent damage to eye tissue by free radicals. In addition, they have an antimicrobial effect and promote the healing of injuries.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
ZRENADOL CHERNIKA is applied in the following:
- as a part of measures to prevent the deterioration of vision organs;
- at high visual load (work at the computer, long time driving or in bright sunlight);
- combination treatment of vascular pathologies of the eye (cataract, glaucoma, myopia);
- for patients with impaired twilight vision (“night blindness”).
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN BETWEEN 3 AND 7 YEARS OF AGE
CHILDREN BETWEEN 7 AND 14 YEARS OF AGE
ADULTS AND CHILDREN ABOVE 14 YEARS OF AGE
1.5-2.0 ml 2 times a day
2.5 ml 2 times a day
5.0 ml 2 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated after a weak.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.