FEMALIFE
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Lemon balm (Melissa officinalis L.)
The common fennel (Fructus Foeniculi)
Breckland thyme (Herba Serpylli)
Anise fruits (Fructus Pimpinella anisum L.)
EFFECT OF THE MEDICINE
- one of the few natural products developed to preserve and maintain breastfeeding with insufficient lactation.
- contributes to the extension of the period of natural breastfeeding, leads to an early recovery of the organism of the breastfeeding woman in the postpartum period and supports it during feeding.
- The basis of FEMALIFE is herbs and their seeds, which have long been used in folk medicine with a lack of milk from breastfeeding women.
SIDE EFFECTS
Usually, side effects do not occur, in most cases, the medication is well tolerated.
There may be allergic reactions (itching, rash), as well as nausea, headache, a feeling of bitterness in the mouth. Use of the medicine should be discontinued when hypersensitivity reactions occur and consult a doctor.
SPECIAL INSTRUCTIONS
After opening the vile, store in a cool place.
INTERACTION WITH OTHER MEDICATIONS
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Increases lactation and replenishes the lack of vitamins and minerals in the body of a woman during breastfeeding;
- Possesses antibacterial and pronounced antioxidant effect;
- Helps relieve stress and fatigue;
- Strengthens the immune system;
CONTRAINDICATIONS
Hypersensitivity to some components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
INSIDE
2 tablets 2-3 times a day, half an hour before meals.
Duration is not limited (usually for the entire period of breastfeeding the baby).
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
SLABIZIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with a protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
Aloe Extract (Aloe arborescens Mill.)
Rhubarb Extract (Rheum L.)
Plantains Extract (Plantago L.)
Egyptian senna (Senna Alexandrina)
EFFECT OF THE MEDICINE
- Slabizin – anthraglycoside containing a preparation of plant origin, possesses a laxative effect coming in 8-10 hours.
- The laxative effect is due to the effect on the receptors of the large intestine, which increases peristalsis.
- It transforms in the digestive tract, begins to irritate the walls of the large intestine, stimulates its work, but it does not cause spasms.
- The medicine is designed to purify the intestines and restore healthy microflora.
- Promotes the reproduction of healthy and necessary for normal functioning of microflora: enterococci, bifidobacteria, lactobacilli, etc.
SPECIAL INSTRUCTIONS
Shake the vial before using it.
It is not recommended to use medicine for more than 2 weeks.
After taking the medicine, urine can acquire a yellow-brown or red-lilac color.
With caution: it is prescribed for liver and/or kidney diseases, as well as during pregnancy and lactation, in conditions after cavitary operations, as well as children’s age (up to 6 years) based on the dosing regimen.
SIDE EFFECTS
The laxative effect of the medicine may be accompanied by colic pain in the abdomen and flatulence.
With prolonged use, particularly at high doses, there could be possible violations of water-electrolyte metabolism, albuminuria, hematuria, melanin deposition in the intestinal mucosa, nausea, vomiting, diarrhea, urinary discoloration, skin rash, seizures, vascular collapse, fatigue,
and confusion.
INTERACTION WITH OTHER MEDICATIONS
With prolonged use of Slabizin in high doses, it is possible to increase the action of cardiac glycosides and influence the effect of antiarrhythmic medicines in connection with the possibility of developing hypokalemia.
With simultaneous use with thiazide diuretics, glucocorticoids, licorice root preparations, the risk of hypokalemia increases.
INDICATIONS
The medicine Slabizin is prescribed for:
- Crohn’s disease;
- chronic and acute constipation (regulation of the physiological rhythm of emptying the large intestine);
- constipation caused by hypotension and sluggish peristalsis of the large intestine;
- chronic spasmodic colitis;
- regulation of stool with hemorrhoids, proctitis, anal fissures;
- softening of the stool for medical purposes (with hemorrhoids, conditions after surgery on the large intestine and in the anal area).
CONTRAINDICATIONS
- chronic intestinal obstruction;
- acute stomach syndrome;
- appendicitis;
- intestinal obstruction;
- abdominal pain of unknown origin;
- gastrointestinal and uterine bleeding;
- women during critical days and in the period of gestation;
- violations of water-electrolyte exchange.
- hypersensitivity to one or more of the components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Slabizin is taken orally, usually 1 time per day in the evening before bedtime:
ADULTS AND CHILDREN OVER 12 YEARS OF AGE
CHILDREN BETWEEN 6-12 YEARS OF AGE
10-15 ml for each intake. In the absence of the effect, the dose can be increased to 20-25 ml
5-10ml. for each intake and, if necessary, the dose can be increased up to 15ml. for each intake
In the process of selection, the same dose should be taken for several days and gradually increased by 5 ml. syrup. If defecation does not occur after reaching the maximum dose within 3 days, one needs to see a doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
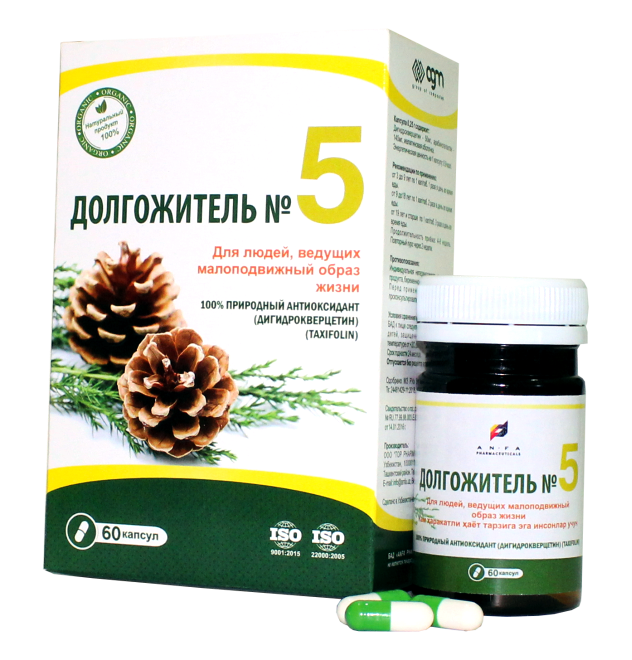
DOLGOJITEL №5
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL №5 – FOR PEOPLE WITH PHYSICAL DISABILITIES
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 5, 0.25 gr. in each capsules, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 92% | 50 mg. |
| Arabinogalactan | 140 mg. |
| Starch | 60 mg. |
EFFECT OF THE MEDICINE
- The preparation is intended for people with physical disabilities and for those who have to lead a sedentary lifestyle.
- Dihydroquercetin and arabinogalactan help to ensure the normal functioning of the body, deprived of the natural possibilities.
- This complex increases vitality, thereby improving the quality of life and increasing its endurance.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
As a means of prevention:
During immunity disorders:
- recommended as a tonic to enhance the body’s defenses;
- increases the body’s resistance to infectious diseases and the harmful effects of the environment.
During exacerbation and complication of inflammatory diseases:
- reduces the severity of inflammatory processes;
- staves off exacerbations and recurrence of chronic inflammatory diseases.
In cardiovascular diseases:
- contributes to the normalization of blood pressure;
- improves blood microcirculation and cerebral circulation;
- reduces the effects of hypoxia and ischemia.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №4
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL №4 – FOR GASTROINTESTINAL TRACT / HEPATOPROTECTOR
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 4, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 95% | 25 mg. |
| Arabinogalactan | 120 mg. |
| Starch | 105 mg. |
EFFECT OF THE MEDICINE
- The preparation with a high content of arabinogalactan is intended for people with gastrointestinal diseases.
- Arabinogalactan stimulates intestinal peristalsis and helps restore normal microflora, thereby improving the work of the gastrointestinal tract.
- Dihydroquercetin (DHQ), due to its antibacterial properties, does away with Helicobacter.
- Moreover, this medication improves the functional state of liver cells, supports its anti-toxic function and is effective for people suffering from constipation.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
As part of complex therapy for diseases of the gastrointestinal tract and liver:
In dysbacteriosis:
- improves nutrition and growth of bifidobacteria and lactobacilli;
- promotes splitting, absorption and assimilation of nutrients in the gastrointestinal tract.
With gastric ulcer and duodenum:
- as part of traditional treatment, it leads to a more rapid and effective reduction of pain, dyspeptic disorders (nausea, vomiting, heartburn, appetite disturbances, feeling of an unpleasant taste in the mouth, flatulence);
- tends to have a protective effect on the mucous membrane;
- contributes to the healing of gastric and duodenal ulcers.
During gastritis:
- improves regeneration of the gastric mucosa, reduces the severity of inflammatory processes.
In the course of liver dysfunction when it is impaired:
- promotes the regeneration of liver cells and stimulates their biosynthetic function;
- reduces the effect of toxins on the liver cells;
- eliminates the symptoms of biliary dyskinesia.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №3
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL № 3 – FOR CARDIOVASCULAR SYSTEM
COMPOSITION AND DOSAGE FORM
DOLGOJITEL №3, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with a protective holographic label; (2) – instruction for use;
Composition:
| Dihydroquercetin (Taxifolin) 95% | 50 mg. |
| Acidum ascorbinicum | 125 mg. |
| Starch | 75 mg. |
EFFECT OF THE MEDICINE
- strengthens the walls of blood vessels and capillaries, increasing their strength and elasticity;
- increases blood flow velocity, while improving microcirculation and the tone of the capillary bed;
- prevents the formation of internal blood clots, which is the cause of many cardiovascular diseases;
- improves the rheological properties of blood, its viscosity, and clotting, weakens the aggregation of red blood cells, improves their deformability in diseases involving increased blood viscosity syndrome (cerebral ischemia of the brain and heart, hypertension, diabetes);
- evinces membrane stabilizing activity, reducing the severity of the inflammatory process;
- reduces the level of cholesterol, lipids in the liver and serum;
- inhibits age-related changes in blood vessels, while slowing the progression of vascular diseases;
- reduces blood clots and the risk of progression of diabetic vascular complications, positively affecting the permeability of blood vessels.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
In the complex therapy of cardiovascular diseases:
In atherosclerosis:
- During the course of treatment with Dolgojitel №3, the total cholesterol concentration decreases, as well as the coefficient of atherogenic serum decreases, and triglyceride levels in the blood reduces. This helps to reduce the clinical presentations of atherosclerosis.
In ischemic heart disease, heart failure, heart rhythm disorders:
- Dolgojitel №3 helps to improve the rheological properties of blood, improves the condition of the membranes, increases their deformability, improves hemodynamic parameters.
During arterial hypertension of I and II degree.
- Under the influence of Dolgojitel №3, blood pressure normalizes, the amplitude of its daily fluctuations decreases, vascular tone ameliorates, cerebral circulation improves. As a result, it decreases headache and dizziness.
For prevention and in the period of rehabilitation after a stroke and heart attack.
- Dolgojitel №3 restores microcirculation, helps to improve venous outflow, reduces hypoxia, renders a positive effect on metabolic processes in cells. Moreover, it helps to prevent the recurrence of the disease.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
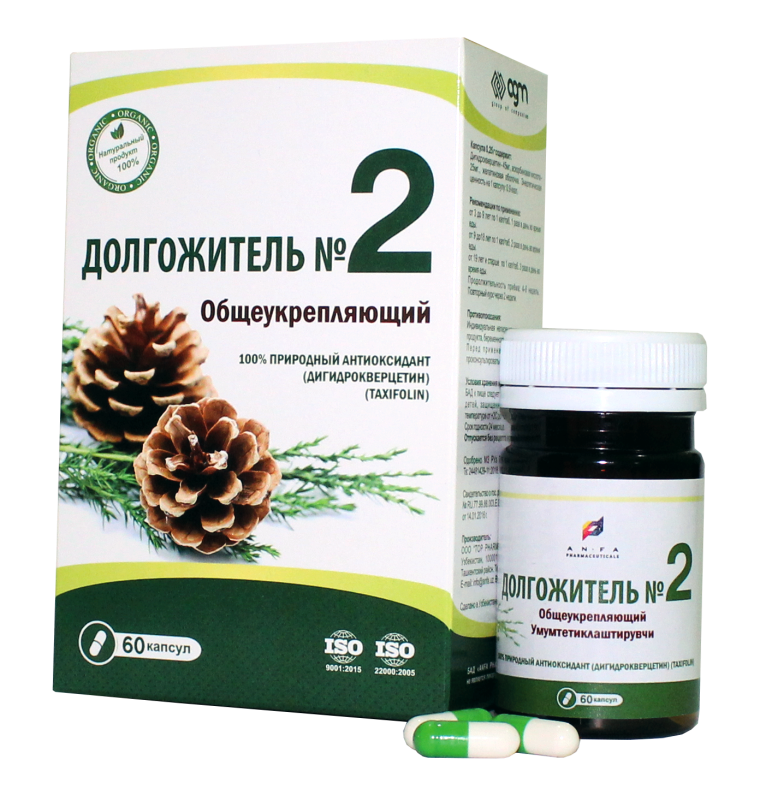
DOLGOJITEL №2
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL №2 – RESTORATIVE
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 2, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 92% | 45 mg. |
| Acidum ascorbinicum | 25 mg. |
| Starch | 180 mg. |
INDICATIONS
- Restorative agent, indispensable for any person in a modern rhythm of life;
- General languor, a decrease in physical and intellectual performance, absent-mindedness, depression – these are signs of inadequate tissue supply with nutrients and oxygen as well as an insufficient rate of excretion of degraded products.
- Dihydroquercetin and its derivatives, through strengthening tissues of blood vessels, contribute to the restoration of their transport function and the disposal of “manager’s syndrome”;
- Taxifolin is an excellent preventive agent for slowing down the aging process in the body.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
In case of immunity disorders:
- It is recommended as a tonic to strengthen the body’s defenses, resistance to infectious diseases and the harmful effects of the environment.
In exacerbations and complications of inflammatory diseases:
- resistance improves, the severity of the inflammatory process reduces, the exacerbation and recurrence of chronic inflammatory diseases stave off.
In the course of liver dysfunction when it is impaired:
- the functional condition of the liver cells improves, and its antitoxic function will be maintained.
In varicose veins, phlebothrombosis, with post-thrombophlebitic syndrome:
- strengthens the venous walls, improves venous blood flow, prevents thrombosis and reduces edema.
In the complications of diabetes:
- improves microcirculation, strengthens the capillary bed, reduces insulin resistance, and improves the quality of life.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №1
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
ДОЛГОЖИТЕЛЬ № 1 – ДЛЯ СПОРТСМЕНОВ
СОСТАВ И ФОРМА ВЫПУСКА
Долгожитель №1 по 0,25 гр. в капсулах, расфасовано в оригинальных флаконах с защитной пломбой по 60 шт. – (1) пачки картонные с защитной фирменной голографической наклейкой; – (2) аннотация.
Состав:
| Дигидрокверцетин (Taxifolin) 95% | 40 мг. |
| Аробиногалактан | 80 мг. |
| Аскорбиновая кислота | 25 мг. |
| Крахмал | 105 мг. |
Показания
- для спортсменов и людей, чья жизнь связана с большими физическими и эмоциональными нагрузками;
- повышает выносливость;
- улучшает работоспособность;
- помогает максимально сконцентрироваться на достижении результата.
ПОБОЧНОЕ ДЕЙСТВИЕ
Обычно не возникает, в подавляющем большинстве случаев препарат переносится хорошо.
При появлении побочных эффектов, в том числе не указанных в инструкции, следует прекратить применение препарата и обратиться к врачу.
Противопоказания
Индивидуальная непереносимость компонентов продукта, беременность, кормление грудью.
ВЗАИМОДЕЙСТВИЕ С ДРУГИМИ ПРЕПАРАТАМИ
При назначении с другими лекарственными средствами отрицательных эффектов не выявлено.
Способ применения и дозы
Длительность курса лечения и дозы препарата определяет лечащий врач индивидуально для каждого пациента.
ОТ 9 ДО 18 ЛЕТ
ОТ 19 ЛЕТ И СТАРШЕ
по 1 кап. 2 раза в день во время еды.
по 1 кап. 3 раза в день во время еды.
Продолжительность приёма
4-8 недель.
Повторный курс через 2 недели.
Перед применением рекомендуется проконсультироваться с врачом.
Условия хранения и сроки годности
Препарат следует хранить в недоступном для детей месте, защищенном от света, при температуре от + 2° C до + 25° C.
Срок годности: 24 месяцев
Не является лекарственным средством.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №1
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL № 1 – FOR ATHLETES
COMPOSITION AND DOSAGE FORM
DOLGOJITEL №1, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with a protective holographic label; (2) – instruction for use;
Composition:
| Dihydroquercetin (Taxifolin) 95% | 40 mg. |
| Arabinogalactan | 80 mg. |
| Acidum ascorbinicum | 25 mg. |
| Starch | 105 mg. |
INDICATIONS
- for athletes and people whose life is associated with great physical and emotional stress;
- increases endurance and stamina;
- improves efficiency;
- helps to concentrate as much as possible on achieving a result.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the agent.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label,
(2) – instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic, diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Biologic corrector of carbohydrate metabolism;
- Normalizes carbohydrate metabolism and lowers blood sugar levels;
- Compensates energy deficit;
- When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
- In Obesity, medicine contributes to the reduction and stabilization of weight;
- Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
INSIDE
2 tablets 3 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
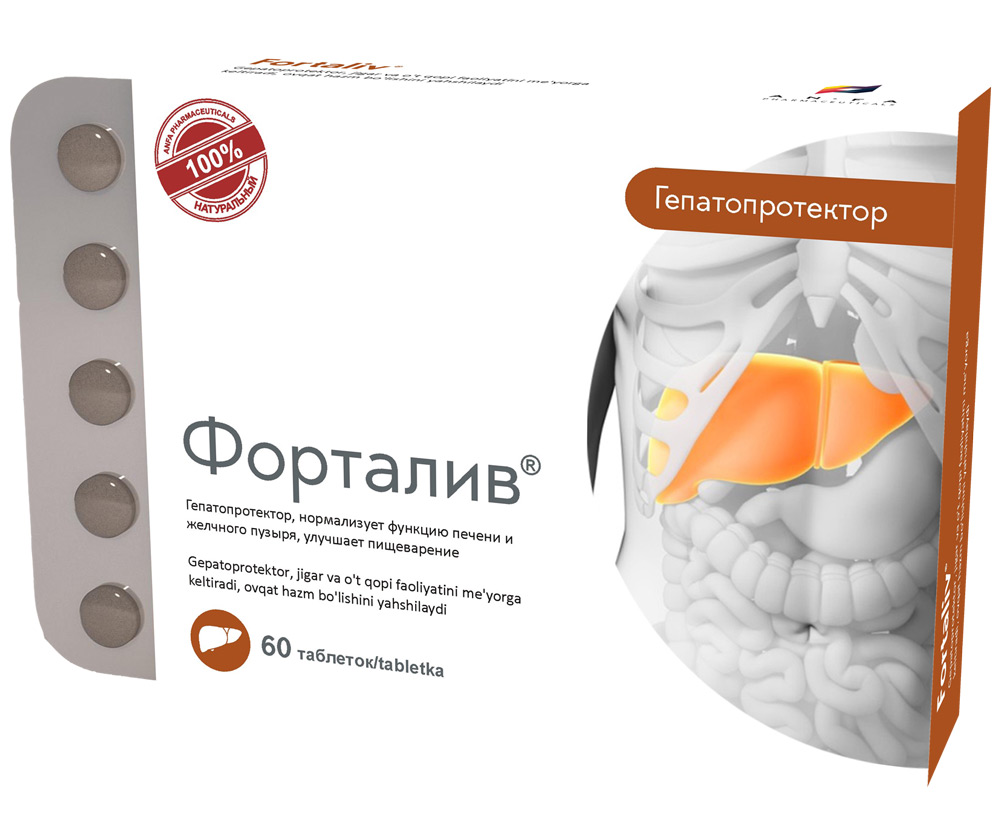
FORTALIV
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Sandy Everlasting (Helichrýsum arenárium L)
Common Agrimony (Agrimónia eupatória L.)
Corn Silk (Stigmata Maydis L.)
Tansy (Tanacétum vulgáre L.)
Milk Thistle (Sílybum mariánum L.)
EFFECT OF THE MEDICINE
- Combination of herbal hepatoprotector;
- Promotes the regeneration of liver cells and stimulates their biosynthetic function;
- Reduces the impact of toxins on liver cells and eliminates biliary dyskinesia symptoms;
- Improves appetite and normalizes digestion;
- Possesses choleretic, anti-oxidant, anti-inflammatory and a slight diuretic effect.
SIDE EFFECTS
Usually, there are no side effects, in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATIONs
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine is prescribed for:
- chronic and acute (during re-convalescence);
- hepatitis of various etiologies (viral, drugs, alcohol, etc.);
- Cirrhosis of the liver and pre-cirrhosis states (in the complex therapy);
- Fatty liver Disease and biliary dyskinesia;
- Cholecystitis.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 YEARS OF AGE AND ABOVE
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal.
2 tablets 3 times a day 5-10 minutes before the meal.
The course of treatment in chronic diseases is usually 20 – 30 days. If necessary the treatment course can be repeated.
OVERDOSE
The cases of FORTALIV overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicineshould be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.