LIPOVITILIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND FORM OF ISSUE
The liquid is light brown in color.
Original bottles of 30 ml with a special nozzle for spraying.
– Packets made of cardboard with protective holographic sticker;
– Instructions for use.
30 ml. of the product contains:
Phospholipids from bovine brain
0.83 gr
Cholesterol
0.15 gr
Copper sulphate
0,003 gr
L-tirozin
0,003 gr
a-tokoferol
0,016 gr
Zinc sulphate
0,003 gr
Water
29 gr
EFFECT OF THE MEDICINE
– Enhances the synthesis of melanin and re-pigmentation of the skin affected areas;
– Regulates the metabolism of the skin;
– Protects it from dehydration;
– Retains elasticity of the skin;
– Neutralizes toxic free radicals;
– Has a rejuvenating effect;
SPECIAL INSTRUCTIONS AND PRECAUTIONS:
Use of the medicine should be discontinued when the burn or allergic reaction occurs.
INTERACTION WITH OTHER MEDICATION
Interaction with Other Medication: When used in the complex therapy increases the antioxidant, membrane modifying and stimulating melanogenesis action.
APPLICATION
The medicine improves the condition of the skin in vitiligo and other cosmetic defects.
ADMINISTRATION AND DOSAGE
The medicine is for external use only.
Apply on the affected area 2 times a day.
The treatment course is 6-8 weeks.
CONTRAINDICATIONS:
Hypersensitivity to the components of the medicine.
SIDE EFFECTS
Not revealed.
STORAGE CONDITIONS
The medicine should be stored out of reach of children, protected from light, in sealed vials at a temperature up to + 10°C.
STORAGE CONDITIONS:
Keep in a place protected from light and inaccessible to children in hermetically sealed bottles at temperatures up to + 10 ° C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
IMMUNOROK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
| Composition: | |
| Rose root Extract (Rhodiola rosea) | |
| Peppermint Extract (Méntha piperíta) | |
| Coneflower Extract (Echinacea purpurea (L.) | |
| Liquorice Extract (Glycyrrhiza glabra) | |
| Rose Hips Extract (Rosa majalis) | |
| Elecampane Extract (Inula helenium) |
EFFECT OF THE MEDICINE
- Immunomodulatory effect of the medicine is achieved by activation of humoral and cellular immunity;
- Effective against influenza viruses, parainfluenza, herpes viruses, enteroviruses, rotavirus;
- Stimulates the bactericidal activity of neutrophils;
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
The usage of the medicine must be informed to the doctor before the surgery.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Asthenic state, neurotic and somatoform disorders;
- Relieving symptoms of uncomplicated viral and bacterial diseases;
- The treatment of acute and chronic herpes and cytomegalovirus infections;
- Reduced physical performance;
- Prevention and treatment of infections in immunodeficient states;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN FROM 1 TO 6 YEARS OF AGE
CHILDREN FROM 7 TO 12 YEARS OF AGE
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
1.0-1.5 ml 3 times a day
1.5-2.5 ml 3 times a day
2.5-5.0 ml 3 times a day
For peroral use, 10-15 minutes before the meal.
The medicine should be taken at least 15 days to achieve a therapeutic effect.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
NATUROSED
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Peppermint (Méntha piperíta)
Oregano (Origanum Vulgare L)
Common Valerian (Valeriána officinális L.)
Cowberry Leaves (Folium vitis-idaea L.)
Creeping Thyme (Thymus serpyllum L.)
Common Motherwort (Leonurus L.)
Hawthorn (Crataegus sanguinea)
EFFECT OF THE MEDICINE
- improves the central nervous system functioning;
- contributes to the improvement of the autonomic nervous system;
- improves the psycho-emotional stability of the body, normalizes sleep;
- contributes to the preservation of peace stressful situations;
- strengthens the immune system and increases the body’s defenses.
SIDE EFFECTS
Usually does not occur, in most cases, the drug is tolerated well.
In the case of individual hypersensitivity to one or more of the components of the drug, allergic reactions, dyspepsia disorders on the part of the gastrointestinal tract are possible.
When developing a hypersensitivity reaction, the drug should be discontinued.
INTERACTION WITH OTHER PREPARATIONS
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Functional disorders of the central and autonomic nervous system;
- The state of prolonged stress, significant mental and emotional stress;
- Irritability, emotional lability;
- Chronic fatigue, overwork;
- Sleep disturbances;
- Cardioneurosis;
- Psychosomatic disorders;
- Climacteric syndrome;
CONTRAINDICATIONS
Hypersensitivity to one or more of the components of the medicine.
DOSage
The duration of treatment and the dose are determined by a physician for each patient individually.
CHILDREN FROM 3 YEARS OF AGE
ADULTS
1 tablet 3 times a day.
1-2 tablets 3 times a day.
In stationary conditions, as an auxiliary sedative effect, 1 tablet 3 times a day.
Tablets are taken 10-15 minutes before meals.
Usually, for chronic diseases, the course of treatment is 20 – 30 days.
If necessary, a second course is possible.
OVERDOSE
No cases of overdose of Naturosed have been detected.
STORAGE CONDITIONS
The drug should be stored out of reach of children, protected from light, at a temperature of +2 ° C to + 25 ° C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
ALLERGYDON
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
Borage Leaf extract (Borago officinalis L.),
Violet Extract (Viola arvensis),
Dandelion Extract (Taraxacum officinale),
Chamomile Extract (Matricāria chamomīlla L).
EFFECT OF THE MEDICINE
The medicine has an anti-inflammatory and restorative effect on the body, purifies blood from allergens, normalizes the activity of internal organs:
– Strengthens immunity;
– Increases the resistance of the body to allergens;
– Protects the liver, which bears the main load with allergic reactions;
– Removes swelling, redness, rashes on the skin;
– Prevents the appearance of lacrimation, the onset of cold and other symptoms of allergic reactions;
– Normalizes the activity of the urinary system, promotes rapid removal of allergens from the body;
– Improves the function of the digestive system;
– Normalizes the work of the nervous system;
– Supplies the body with the necessary vitamins and microelements;
INDICATIONS
The medicine Allergydon is prescribed for:
- Seasonal allergies (pollinosis), food and medicine allergies;
- Allergic dermatological diseases (itching dermatoses, hives, psoriasis, atopic dermatitis, allergic eczema);
- Allergic diseases of the respiratory system (allergic bronchitis, bronchial asthma, allergic rhinitis)
- Allergic conjunctivitis.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATIONS
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Allergydon is recommended to take:
ADULTS
CHILDREN BELOW 6 YEARS OF AGE
CHILDREN BETWEEN 6 AND 12 YEARS OF AGE
15-20 ml., 3 times a day with a meal
2,5 – 5 ml., 2-3 times a day with a meal
5-10 ml., 2-3 times a day with a meal
Duration of intake – from one to three months, the course of therapy can be repeated one month after the end of the previous course.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
MIGRENAZ-L
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
| Composition: | |
| Valerian Extract (Valeriána officinális) | |
| Brandy Mint Extract (Méntha piperíta) | |
| Ginkgo Biloba Extract (Gīnkgo) | |
| May Lily Extract (Convallária majális) | |
| Field Caraway Extract (Fructus Carvi) | |
| Blood-Red Hawthorn Extract (Crataégus sanguínea) |
EFFECT OF THE MEDICINE
- improves cerebral circulation and brain supply;
- affects the metabolism in cells, the rheological properties of blood and improves peripheral circulation and microcirculation;
- possesses a pronounced anti-edematous effect at the level of the brain and in peripheral tissues;
- anti-stress action manifests itself in the normalization of post-stress behavior, somatovegetative disorders, restoration of sleep-wake cycles, disrupted learning and memory processes, reduction of dystrophic and
morphological changes in various structures of the brain.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
The usage of the medicine must be informed to the doctor before the surgery.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine MIGRENAZ – L is prescribed for:
– acute and chronic disorders of cerebral circulation;
– light craniocerebral trauma; (light concussion of the brain)
– syndrome of vegetative-vascular dystonia (VSD, neurocirculatory dysfunction);
– light cognitive impairment of atherosclerotic origin;
– memory disorders and mental activity;
– dizziness, noise in the ears, a sense of fear;
– violation of peripheral circulation and microcirculation (including arteriopathy of the lower extremities and pediatric orthopedics), Raynaud’s syndrome.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN BETWEEN 4 AND 12 YEARS OF AGE
ADULTS AND CHILDREN OVER 12 YEARS OF AGE
1 tablet 3 times a day
1 tablet 3 times a day
For peroral use, 5-10 minutes before the meal.
The course of treatment is at least 3 months (especially in elderly patients).
Courses can be repeated after consultation with the doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
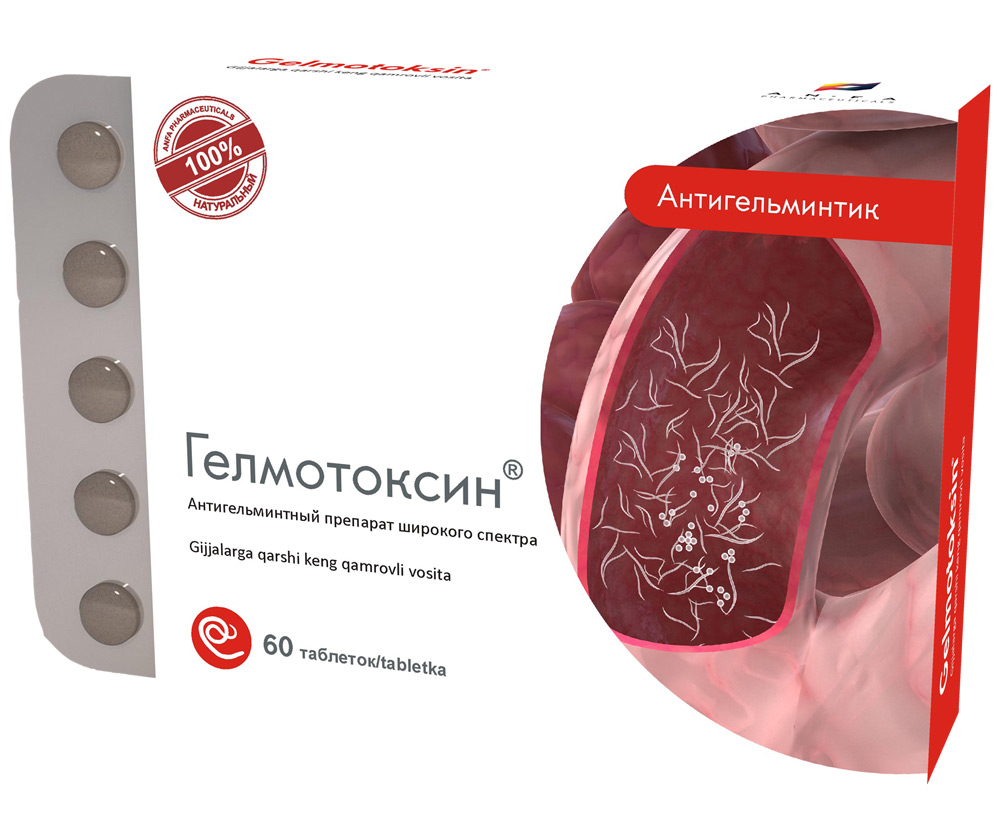
HELMOTOXIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective
holographic label, (2) – instruction for use.
Composition:
Tansy (Tanacétum vulgáre L.)
Elecampane Inula (Inula helenium L.)
Alexandrine senna (Cassia Acutifolia)
Common hop (Húmulus lúpulus L.)
Pumpkin (Cucúrbita pépo L.)
Garlic (Állium satívum L.)
Pharmacological properties
Tansy has antihelminthic (against ascarids and seatworms), giardicidal, choleretic, antispasmodic and astringent action. Increases appetite, the acidity of gastric fluid, improves digestion, has a bactericidal and bacteriostatic effect, has insecticidal properties.
Elecampane Inula acts as an antihelminthic agent (especially in ascariasis), normalizes secretion and motor function, and reduces inflammation activity in the digestive tract.
Alexandrine senna has a laxative effect, so all drugs are prescribed for the night. Administration of drug is not accompanied by cramping pains in the bowel and loose stool which greatly differentiates the drug from other
laxatives and allows extensive use of therapeutic doses in pediatric and geriatric practice.
Common hop has a calming, sedative effect. Its bitter substances improve digestion. The sum of active compounds has a bactericidal effect.
Pumpkin seeds have long been known in traditional medicine, their pharmacological properties are confirmed both experimentally and clinically. Seeds are used against a variety of tapeworms (bovine, porcine,
and dwarf tapeworms, broad tapeworm) and pinworms.
Garlic stimulates the appetite, enhances the excretion of enzymes in the digestive organs, promotes better digestion and absorption of food. Essential oil of garlic has a strong bactericidal, disinfectant action and at
the same time increases the human body’s resistance to pathogens.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated. In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the
gastrointestinal tract.
Use of the drug should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Round-worms (pinworm, ascaris)
- Tapeworms
- Hymenolepiasis
- Giardiasis (in the complex therapy)
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
CHILDREN FROM 3 YEARS OLD AND ABOVE
2 tablets 3 times a day 10-15 minutes before the meal
1 tablet 3 times a day 10-15 minutes before the meal
The course of treatment is usually 21-28 days.
It is recommended to strictly follow a diet. If necessary the treatment course can be repeated.
OVERDOSE
The cases of GELMOTOKSIN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The drug should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
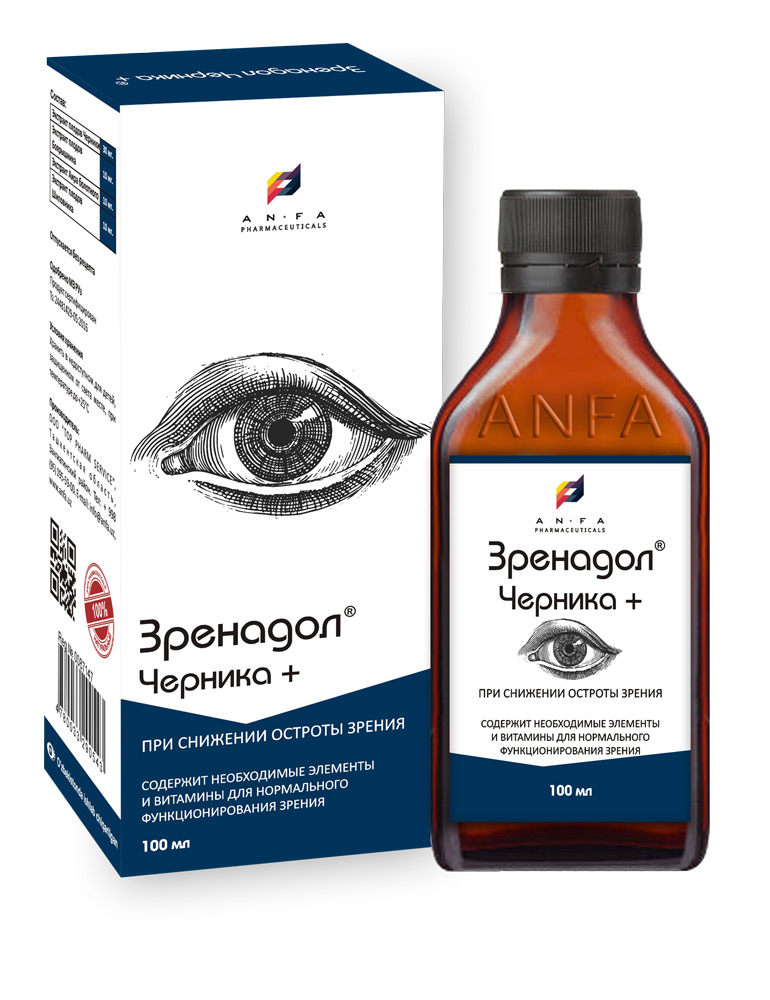
ZRENODOL CHERNIKA+
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts and their fruits, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
| Composition: | |
| Blueberry Fruit Extract (Vaccinium myrtillus) | |
| Hawthorn Fruit Extract (Crataegus) | |
| Sweet flag Extract (Acorus calamus) | |
| Rose Hip Extract (Rosa majalis) |
EFFECT OF THE MEDICINE
- ZRENADOL CHERNIKA+ protects the eyes from fatigue, irritation, and vision weakening;
- improves dark adaptation;
- reduces the permeability of capillaries, strengthens the walls of blood vessels, including the vessels of the eye fundus, reduces swelling and redness;
- supports visual organs with essential nutrients – vitamins and micronutrients;
- Tends to have a positive effect on the functional status of the visual organs associated with the deficiency of vitamins and micronutrients, has a health-promoting effect and is a natural product.
- Blueberry’s anthocyanosides contribute to the regeneration of the photosensitive pigment of the retina, increasing its sensitivity to changes in light intensity, improving visual acuity during low-light conditions; contributes to the improvement of dark adaptation.
- Active substances of Blueberry improve the flexibility of cell membranes and help to enhance blood flow to the retina.
- Blueberry extract and vitamin C (the main component of rose hips) with bioflavonoids promote the blood circulation in the vision organs, reduce intraocular pressure and have strong antioxidant properties, i.e. prevent damage to eye tissue by free radicals. In addition, they have an antimicrobial effect and promote the healing of injuries.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
ZRENADOL CHERNIKA is applied in the following:
- as a part of measures to prevent the deterioration of vision organs;
- at high visual load (work at the computer, long time driving or in bright sunlight);
- combination treatment of vascular pathologies of the eye (cataract, glaucoma, myopia);
- for patients with impaired twilight vision (“night blindness”).
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN BETWEEN 3 AND 7 YEARS OF AGE
CHILDREN BETWEEN 7 AND 14 YEARS OF AGE
ADULTS AND CHILDREN ABOVE 14 YEARS OF AGE
1.5-2.0 ml 2 times a day
2.5 ml 2 times a day
5.0 ml 2 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated after a weak.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
BRONKHODOKS
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
|
|
||
| Common Sage Extract (Salvia officinalis L.) | ||
| Coltsfoot Extract (Tussilago L) | ||
| Licorice Extract (Glycyrrhiza glabra L.) | ||
| Aloe Extract (Aloe arborescens Mill.) | ||
| Plantain Extract (Plantago L) |
EFFECT OF THE MEDICINE
Bronkhodoks is a combination medicine with antitussive and bronchodilatory action.
– It has a suppressive effect on the cough center without affecting breathing.
– It also has weak bronchospasmolytic and secretolytic action.
– The medicine stimulates respiration, expands bronchi and reduces bronchial mucosa edema.
– It has anti-inflammatory and weak antimicrobial effect.
INDICATIONS
Bronkhodoks is used in combination therapy for the treatment of the followings:
- Acute and chronic bronchitis;
- Tracheobronchitis;
- Bronchial asthma;
- Bronchiectasis;
- Sanitation of a bronchial tree in pre – and postoperative period;
- Atelectasis when mucous plug blocks bronchi;
- Pneumonia;
- Pertussis;
- Tuberculosis.
- To eliminate the symptoms of cough and bronchoconstriction
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
Hyperacidity gastritis. Pregnancy. Lactation.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
CHILDREN BETWEEN 3 AND 12 YEARS OF AGE
5.0-7.5 ml, 3 times a day
2.5-5.0 ml, 3 times a day
For peroral use, 10-15 minutes after the meal.
The course of administration is 14 days
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.