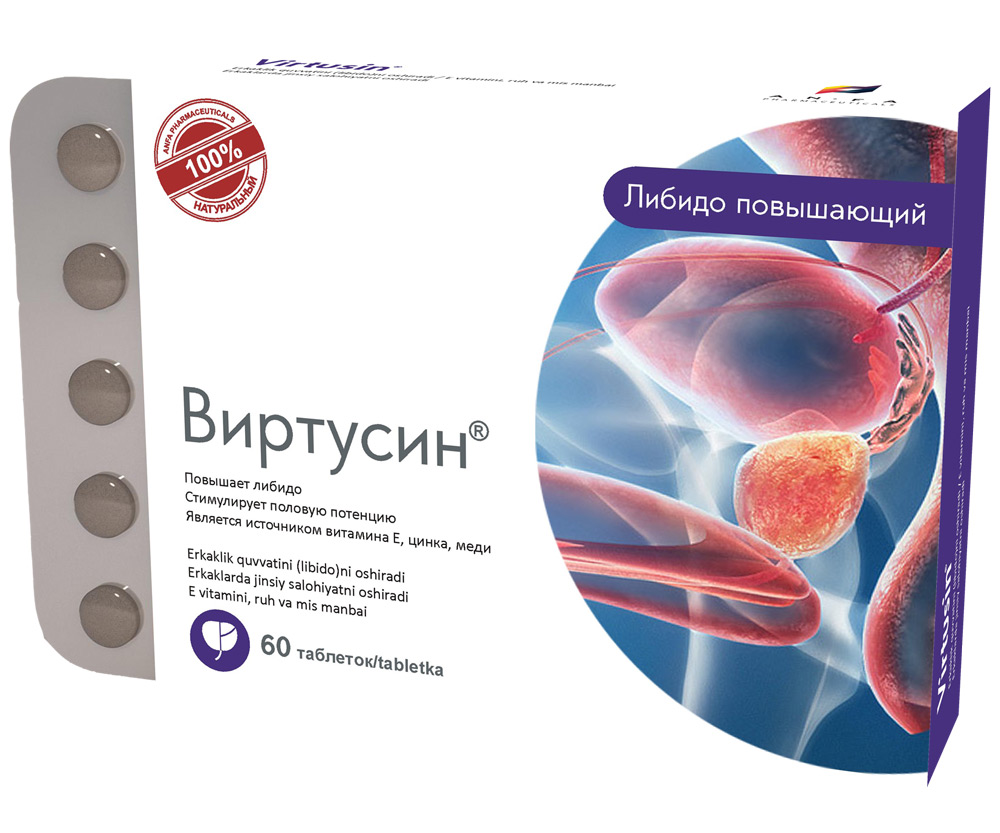
VIRTUSIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective
holographic label, (2) – instruction for use.
Composition:
Anomalous Peony (Paeonia anomala L.)
Rosebay Willow-herb (Chamaenerion angustifolium L.)
Aspen (Populus tremula L.)
Pumpkin (Cucurbita pepo L.)
Horse Chestnut (Aesculus hippocastanum L.)
Common nettle (Urtica dioica L.)
Greater Burdock (Arctium lappa L.)
EFFECT OF THE MEDICINE
- Increases libido, energy, and endurance.
- Normalizes the function of urinary organs in men.
- The medicine is a source of vitamin E, zinc, copper.
- Promotes the improvement and maintenance of the prostate gland function.
- Stimulates sexual potency in men and enhances the reproductive abilities.
INDICATIONS
- Prevention or treatment of acute prostatitis and prostatic adenoma;
- Cystitis;
- Prevention of chronic prostatitis exacerbations;
- Additional long-term therapy after suffering prostatitis;
- Functional disorders of the genitourinary system;
- Reduced erection;
- For normalization of functions and structures of the prostate and improvement of metabolism in its cells.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal
The course of treatment in chronic diseases is usually 30 days.
If necessary the treatment course can be repeated.
SIDE EFFECTS
Usually, there are no side effects. In the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of VIRTUSIN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.