MIGRENAZ – L
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
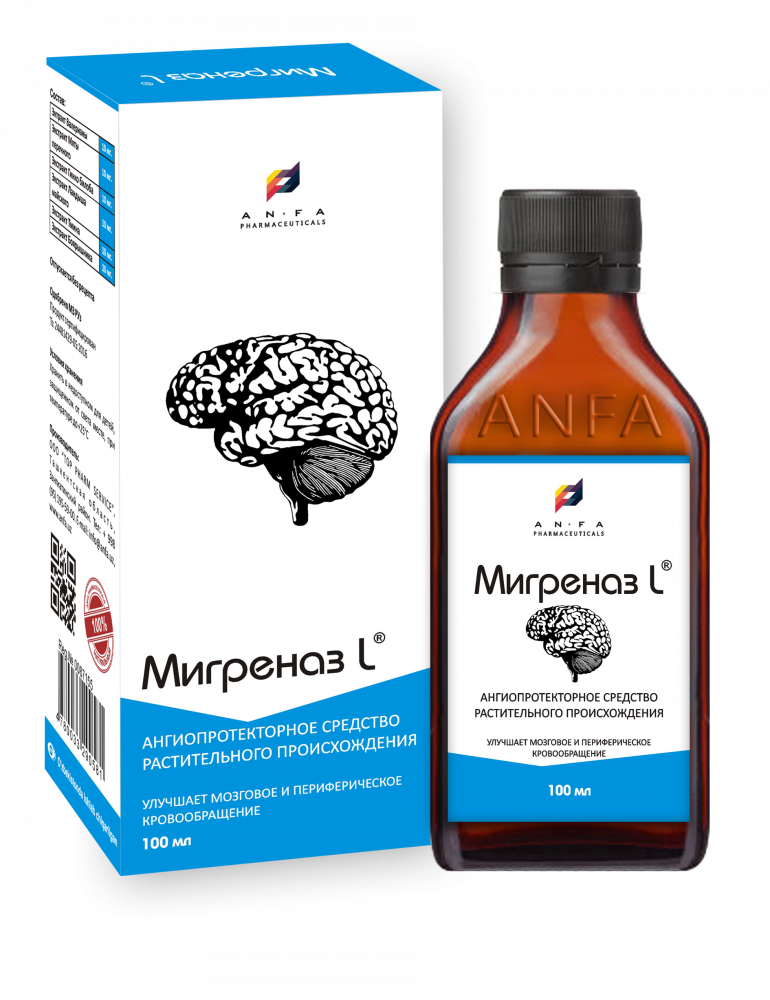
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 100 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Common Valerian Extract (Valeriána officinális)
Brandy Mint Extract (Méntha piperíta)
Ginkgo Biloba Extract (Gīnkgo Biloba)
May Lily Extract (Convallária majális)
Field Caraway Extract (Fructus Carvi)
Blood-Red Hawthorn Extract (Crataégus sanguínea)
EFFECT OF THE MEDICINE
- Improves cerebral blood circulation and brain oxygen supply;
- Affects the metabolism of the cells, blood rheology and improves peripheral circulation and microcirculation;
- Expressed anti-edema effect on the level of the brain and in peripheral tissues;
- It has an anti-stress effect on the normalization of post-stress behavior, somatic-vegetative violations, restoration of the sleep-wake cycles, broken processes of learning and memory, and reducing degenerative
morphological changes in different brain structures.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
The usage of the medicine must be informed to the doctor before the surgery.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
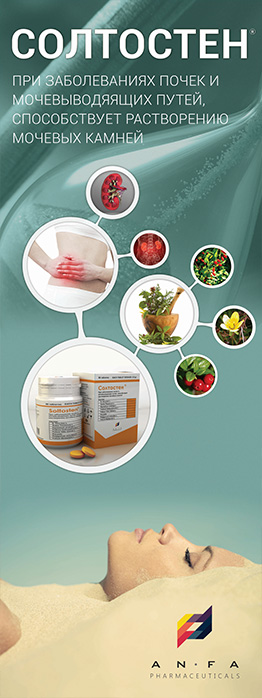
INDICATIONS
Migrenaz – L is used in the treatment and prevention of the following:
- Acute and chronic cerebral circulatory disorders;
- Mild traumatic brain injury (Mild concussion);
- Vegetative dystonia syndrome;
- Mild cognitive impairment of atherosclerotic nature;
- Disorders of memory and mental activity;
- Vertigo, tinnitus, feelings of fear;
- Violation of peripheral blood circulation and microcirculation (including arteriopathy of the lower limbs and pediatric orthopedics), Raynaud’s syndrome.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN FROM 1 TO 3 YEARS OLD
CHILDREN FROM 4 TO 12 YEARS OLD
ADULTS AND CHILDREN FROM 12 YEARS OLD AND ABOVE
1.0-2.5 ml 2-3 times a day
3.0-5.0 ml 2-3 times a day
5.0-7.5 ml 2-3 times a day
For peroral use, 5-10 minutes before the meal. The course of treatment is at least 3 months (especially in elderly patients).
Courses can be repeated after consultation with the doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.