PANKRIAZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKAGING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Myrtleflag (Acorus calamus L.)
Flint Corn (Zea mays L.)
Flax Seeds (lini seed)
Chamomile (Matricaria chamomilla L)
St. John’s-worts (Hypericum perforatum L.).
Marigold Officinalis (Calendula officinalis L.)
Common Wormwood (Artemisia absinthium L)
EFFECT OF THE MEDICINE
The medicine normalizes secretory and motor functions of the choledochopancreatic system and has anti-inflammatory action.
SIDE EFFECTS
There may be rare allergic reactions in the case of individual sensitivity to separate components of the medicine.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine is prescribed in the following cases:
- Lack of the secretory function of the pancreas (chronic pancreatitis – inflammation of the pancreas, cystic fibrosis – an inherited disease characterized by obstruction of the output ducts of the pancreas, respiratory
tract and intestinal glands by viscous secretion, etc.); - Chronic inflammatory and degenerative diseases of the stomach, intestines, liver, gall bladder;
- Status after resection or irradiation of these organs, accompanied by maldigestion, flatulence (gas accumulation in the gut), diarrhea (flux) – in combination therapy;
- State after pancreatectomy (removal of the pancreas);
- Obstruction of the pancreas ducts or biliary tract;
- To improve digestion in patients with the normal function of the gastrointestinal tract in the case of noncompliance to diet, as well as chewing dysfunction, forced prolonged immobilization, sedentary lifestyle;
- Preparation for X-ray and ultrasound examination of the abdominal cavity.
- Cholangitis.
CONTRAINDICATIONS
Acute pancreatitis, chronic pancreatitis in the acute phase.
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADOLESCENTS AND CHILDREN 17 YEARS OLD AND BELOW
ADULTS
1 tablet 3 times a day
2 tablets 3 times a day
Tablets are taken with meals, swallowed as a whole with plenty of non-alkaline liquid (water, fruit juices).
Duration of treatment may vary from a few days (in case of maldigestion due to deviation from the diet) to several months or even years (permanent replacement therapy if necessary).
If necessary the treatment course can be repeated.
OVERDOSE
The cases of the medicine overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
SLABIZIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with a protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
Aloe Extract (Aloe arborescens Mill.)
Rhubarb Extract (Rheum L.)
Plantains Extract (Plantago L.)
Egyptian senna (Senna Alexandrina)
EFFECT OF THE MEDICINE
- Slabizin – anthraglycoside containing a preparation of plant origin, possesses a laxative effect coming in 8-10 hours.
- The laxative effect is due to the effect on the receptors of the large intestine, which increases peristalsis.
- It transforms in the digestive tract, begins to irritate the walls of the large intestine, stimulates its work, but it does not cause spasms.
- The medicine is designed to purify the intestines and restore healthy microflora.
- Promotes the reproduction of healthy and necessary for normal functioning of microflora: enterococci, bifidobacteria, lactobacilli, etc.
SPECIAL INSTRUCTIONS
Shake the vial before using it.
It is not recommended to use medicine for more than 2 weeks.
After taking the medicine, urine can acquire a yellow-brown or red-lilac color.
With caution: it is prescribed for liver and/or kidney diseases, as well as during pregnancy and lactation, in conditions after cavitary operations, as well as children’s age (up to 6 years) based on the dosing regimen.
SIDE EFFECTS
The laxative effect of the medicine may be accompanied by colic pain in the abdomen and flatulence.
With prolonged use, particularly at high doses, there could be possible violations of water-electrolyte metabolism, albuminuria, hematuria, melanin deposition in the intestinal mucosa, nausea, vomiting, diarrhea, urinary discoloration, skin rash, seizures, vascular collapse, fatigue,
and confusion.
INTERACTION WITH OTHER MEDICATIONS
With prolonged use of Slabizin in high doses, it is possible to increase the action of cardiac glycosides and influence the effect of antiarrhythmic medicines in connection with the possibility of developing hypokalemia.
With simultaneous use with thiazide diuretics, glucocorticoids, licorice root preparations, the risk of hypokalemia increases.
INDICATIONS
The medicine Slabizin is prescribed for:
- Crohn’s disease;
- chronic and acute constipation (regulation of the physiological rhythm of emptying the large intestine);
- constipation caused by hypotension and sluggish peristalsis of the large intestine;
- chronic spasmodic colitis;
- regulation of stool with hemorrhoids, proctitis, anal fissures;
- softening of the stool for medical purposes (with hemorrhoids, conditions after surgery on the large intestine and in the anal area).
CONTRAINDICATIONS
- chronic intestinal obstruction;
- acute stomach syndrome;
- appendicitis;
- intestinal obstruction;
- abdominal pain of unknown origin;
- gastrointestinal and uterine bleeding;
- women during critical days and in the period of gestation;
- violations of water-electrolyte exchange.
- hypersensitivity to one or more of the components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Slabizin is taken orally, usually 1 time per day in the evening before bedtime:
ADULTS AND CHILDREN OVER 12 YEARS OF AGE
CHILDREN BETWEEN 6-12 YEARS OF AGE
10-15 ml for each intake. In the absence of the effect, the dose can be increased to 20-25 ml
5-10ml. for each intake and, if necessary, the dose can be increased up to 15ml. for each intake
In the process of selection, the same dose should be taken for several days and gradually increased by 5 ml. syrup. If defecation does not occur after reaching the maximum dose within 3 days, one needs to see a doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №4
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL №4 – FOR GASTROINTESTINAL TRACT / HEPATOPROTECTOR
COMPOSITION AND DOSAGE FORM
DOLGOJITEL № 4, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
| Dihydroquercetin (Taxifolin) 95% | 25 mg. |
| Arabinogalactan | 120 mg. |
| Starch | 105 mg. |
EFFECT OF THE MEDICINE
- The preparation with a high content of arabinogalactan is intended for people with gastrointestinal diseases.
- Arabinogalactan stimulates intestinal peristalsis and helps restore normal microflora, thereby improving the work of the gastrointestinal tract.
- Dihydroquercetin (DHQ), due to its antibacterial properties, does away with Helicobacter.
- Moreover, this medication improves the functional state of liver cells, supports its anti-toxic function and is effective for people suffering from constipation.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the drug.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATION
As part of complex therapy for diseases of the gastrointestinal tract and liver:
In dysbacteriosis:
- improves nutrition and growth of bifidobacteria and lactobacilli;
- promotes splitting, absorption and assimilation of nutrients in the gastrointestinal tract.
With gastric ulcer and duodenum:
- as part of traditional treatment, it leads to a more rapid and effective reduction of pain, dyspeptic disorders (nausea, vomiting, heartburn, appetite disturbances, feeling of an unpleasant taste in the mouth, flatulence);
- tends to have a protective effect on the mucous membrane;
- contributes to the healing of gastric and duodenal ulcers.
During gastritis:
- improves regeneration of the gastric mucosa, reduces the severity of inflammatory processes.
In the course of liver dysfunction when it is impaired:
- promotes the regeneration of liver cells and stimulates their biosynthetic function;
- reduces the effect of toxins on the liver cells;
- eliminates the symptoms of biliary dyskinesia.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 3 AND 9 YEARS OF AGE
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 1 time a day with meals.
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DOLGOJITEL №1
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
DOLGOJITEL № 1 – FOR ATHLETES
COMPOSITION AND DOSAGE FORM
DOLGOJITEL №1, 0.25 gr. in each capsule, 60 pcs of capsules packaged in original bottles with a protective seal. (1) – cardboard pack with a protective holographic label; (2) – instruction for use;
Composition:
| Dihydroquercetin (Taxifolin) 95% | 40 mg. |
| Arabinogalactan | 80 mg. |
| Acidum ascorbinicum | 25 mg. |
| Starch | 105 mg. |
INDICATIONS
- for athletes and people whose life is associated with great physical and emotional stress;
- increases endurance and stamina;
- improves efficiency;
- helps to concentrate as much as possible on achieving a result.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the drug is well tolerated.
Use of the drug should be discontinued when hypersensitivity reactions occur.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the agent.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
Dosage and Administration
The duration of treatment and the dose are determined by a physician for each patient individually.
PATIENTS BETWEEN 9 AND 18 YEARS OF AGE
PATIENTS ABOVE 19 YEARS OF AGE
1 capsule 2 times a day with meals.
1 capsule 3 times a day with meals.
Duration of admission
4-8 weeks.
Repeated course after 2 weeks.
Consult with your doctor before use.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
Not considered as a pharmaceutical medicine.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label,
(2) – instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic, diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Biologic corrector of carbohydrate metabolism;
- Normalizes carbohydrate metabolism and lowers blood sugar levels;
- Compensates energy deficit;
- When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
- In Obesity, medicine contributes to the reduction and stabilization of weight;
- Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
INSIDE
2 tablets 3 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
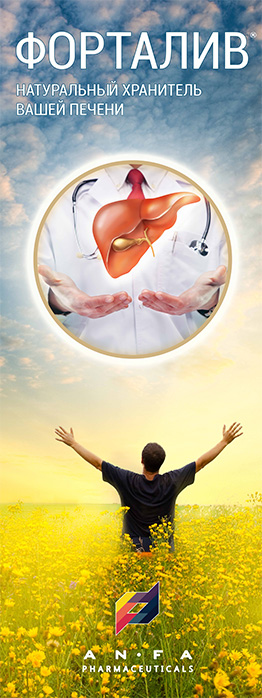
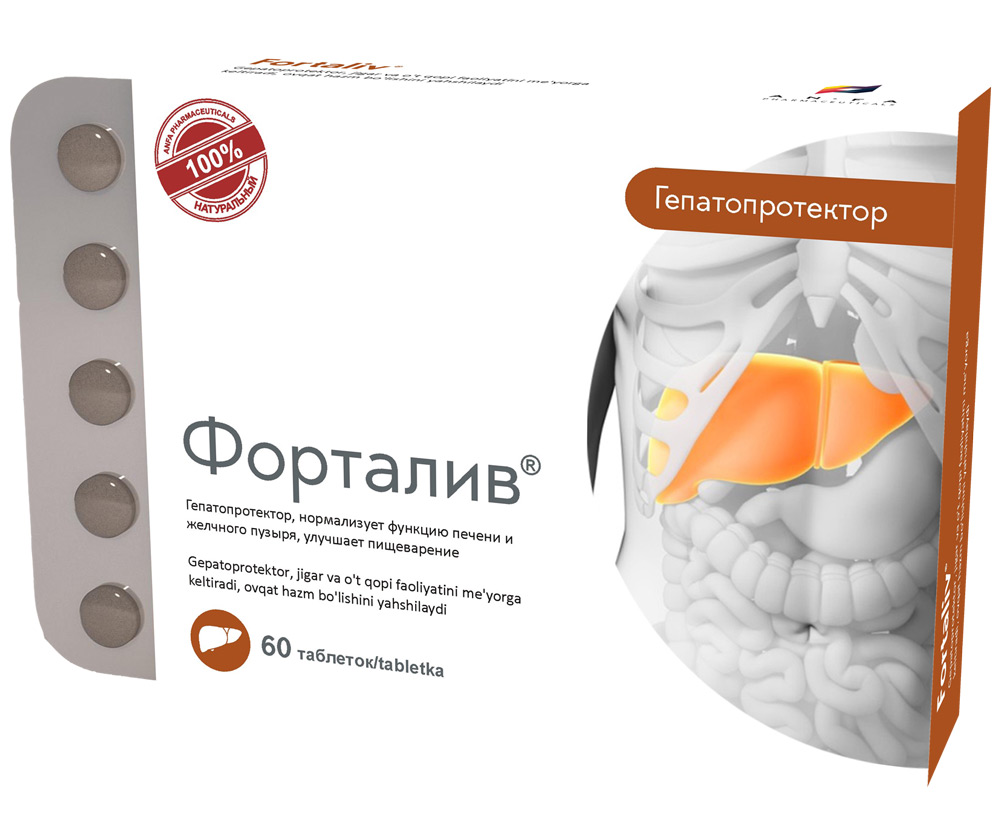
FORTALIV
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Sandy Everlasting (Helichrýsum arenárium L)
Common Agrimony (Agrimónia eupatória L.)
Corn Silk (Stigmata Maydis L.)
Tansy (Tanacétum vulgáre L.)
Milk Thistle (Sílybum mariánum L.)
EFFECT OF THE MEDICINE
- Combination of herbal hepatoprotector;
- Promotes the regeneration of liver cells and stimulates their biosynthetic function;
- Reduces the impact of toxins on liver cells and eliminates biliary dyskinesia symptoms;
- Improves appetite and normalizes digestion;
- Possesses choleretic, anti-oxidant, anti-inflammatory and a slight diuretic effect.
SIDE EFFECTS
Usually, there are no side effects, in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATIONs
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine is prescribed for:
- chronic and acute (during re-convalescence);
- hepatitis of various etiologies (viral, drugs, alcohol, etc.);
- Cirrhosis of the liver and pre-cirrhosis states (in the complex therapy);
- Fatty liver Disease and biliary dyskinesia;
- Cholecystitis.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 YEARS OF AGE AND ABOVE
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal.
2 tablets 3 times a day 5-10 minutes before the meal.
The course of treatment in chronic diseases is usually 20 – 30 days. If necessary the treatment course can be repeated.
OVERDOSE
The cases of FORTALIV overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicineshould be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DEPARAZITOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
Tansy Extract (Tanacetum vulgare L.)
Chamomile Extract (Matricaria Chamomilla L.)
Perforate St John’s-wort Extract (Hypericum L.)
Yarrow Extract (Achillea millefolium L.)
Dwarf everlast (Helichrysum Arenarium L.)
Pumpkin Seed Extract (Cucurbita pepo L)
EFFECT OF THE MEDICINE
DEPARAZITOL — Anthelmintic preparation of a wide spectrum of action. It is most effective against Enterobius vermicularis, Trichuris trichiura, Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus, Strongyloides stercoralis, Taenia solium, Echinococcus granulosus, Echinococcus multilocularis, Trichinella spiralis, Trichinella nativa, Trichinella nelsoni.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation. Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine Deparazitol is prescribed for:
- enterobiasis;
- ascaridosis;
- ancylostomiasis;
- strongyloidiasis;
- trichocephalus;
- trichinosis;
- taeniasis;
- mixed helminthiases.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Deparazitol is recommended to take: Inside with a little amount of water.
CHILDREN OVER 3 YEARS OF AGE:
– when enterobiasis – 5 ml. 1 time per day,
– with mixed helminthiases – in the morning and in the evening 10ml. for 7 days.
If the symptoms persist, it is recommended to repeat the course of treatment after 3 weeks.
ADULTS AND CHILDREN OVER 14 YEARS OF AGE:
– when trichinellosis – on the first day for 10-15 ml. 3 times a day, on the second day for 10-15 ml. 4 times a day, and from 3 to 14 day – 25 ml. 3 times a day.
– with ascariasis, trichocephalosis, ankylostomiasis, teniosis, strongyloidiasis, and mixed helminthiases – in the morning and in the evening 10 ml. for 3 days.
Duration of intake – from one to three months, the course of therapy can be repeated one month after the end of the previous course.
It is recommended that all members of the family be treated at the same time.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic,
diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
– Biologic corrector of carbohydrate metabolism;
– Normalizes carbohydrate metabolism and lowers blood sugar levels;
– Compensates energy deficit;
– When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
– Obesity, contributes to the reduction and stabilization of weight;
– Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
SYRUP
2.5-5.0 ml 3 times a day
The medicine is administered with meals with plenty of water.
Course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
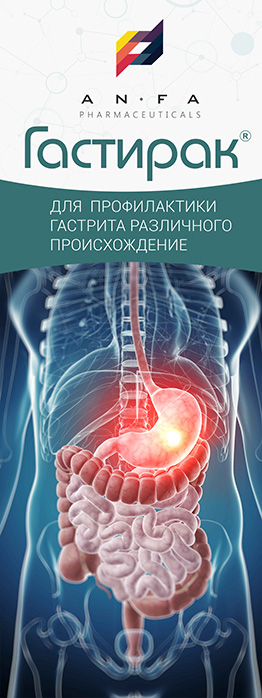
GASTIRAK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Myrtle flag (Acorus cálamus)
Plantain (Plantago L.)
Walnut (Júglans régia)
Aloe (Aloe L.)
Chamomile (Matricaria L.)
Melissa (Melissa offlcinalis L.)
EFFECT OF THE MEDICINE
GASTIRAK is a combined medicine with antacid, astringent, antispasmodic, and laxative properties.
– helps to reduce the activity of pepsin and gastric acidity.
– provides restorative, antibacterial and anti-inflammatory action, forming a protective film on the gastric mucosa.
– the active ingredients of aloe and myrtle flag are characterized by antispasmodic activity, and Melissa has a laxative effect.
INDICATIONS
- Hyperacid gastritis;
- Non-ulcer dyspepsia;
- Gastroesophageal reflux disease;
- Peptic and duodenal ulcer, including ulcer associated with Helicobacter pylori.
- Gastralgia, acid regurgitation;
- Heartburn of different etiology;
- The medicine is also used for the symptomatic treatment of pain in the stomach caused by diet non-compliance, alcohol abuse, smoking and treatment with medicines that are irritating to the gastric mucosa.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
Renal failure.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
CHILDREN BETWEEN 6 AND 12 YEARS OF AGE
1-2 tablets three times a day
1 tablet three times a day
GASTIRAK is recommended to take 30-60 minutes after the meal. Crush the tablets into powder and take with 100 ml of water.
The course of administration is usually from one to three months; treatment can be repeated a month after the end of the previous course.
SIDE EFFECTS
Usually, there are no side effects. In most cases, the medicine is well tolerated. In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of GASTIRAK overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
PROCURIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 30 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Common knotgrass (Polýgonum aviculáre L.)
Burnet Bloodwort (Sanguisórba officinális L.)
Elecampane Inula (Ínula helénium L.)
Perforate St John’s-wort (Hypéricum perforátum L.)
Sandy Everlasting (Helichrýsum arenárium L.)
Walnut (Júglans régia L.)
Horse Sorrel (Rúmex confértus L.)
Peppermint (Méntha piperíta L.)
EFFECT OF THE MEDICINE
- The medicine acts as a combined antidiarrheal, astringent, anti-inflammatory, disinfection action.
- It has a calming, antispasmodic, antiseptic and analgesic effect on the mucosa of the gastrointestinal tract.
- The antidiarrhoeal effect is achieved by reducing of vermicular movement, intestinal and secretory activity of intestinal tract, and accelerates the regeneration of the mucous membranes of the gastrointestinal tract.
- It has antimicrobial activity (action aimed at the destruction of germs); it normalizes the intestinal flora.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
During treatment, fatty foods, alcohol, and melons need to be proscribed from the diet.
It is necessary to follow a diet and short breaks between meals are recommended with a gradual return to the original nature of the diet, drink plenty of fluids, avoid eating spices and limit the reception of flour products.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
If necessary the treatment course can be repeated.
INDICATIONS
- non-specific acute and chronic diarrhea;
- diarrhea associated with allergic reactions;
- diarrhea caused by psycho-emotional factors;
- diarrhea due to changes in diet and eating habits;
- colitis;
- dysbacteriosis after taking broad-spectrum antibiotics (prevention and treatment);
- enteritis, accompanied by diarrhea;
- flatulence;
- the medicine is used as an aid for infectious diarrhea.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 years old and below as a therapeutic enema
CHILDREN 3 years old and above – per oral administraion
ADULTS
2 tablets for 100 ml of water
1 tablet 3 times a day 5-10 minutes before the meal
2 tablets 3 times a day 5-10 minutes before the meal
The duration of treatment depends on the reason of dysbacteriosis development and individual peculiarities of the body.
The course of treatment in chronic diseases is usually 5 – 10 days.
If necessary the treatment course can be repeated.
OVERDOSE
The cases of PROCURIN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.