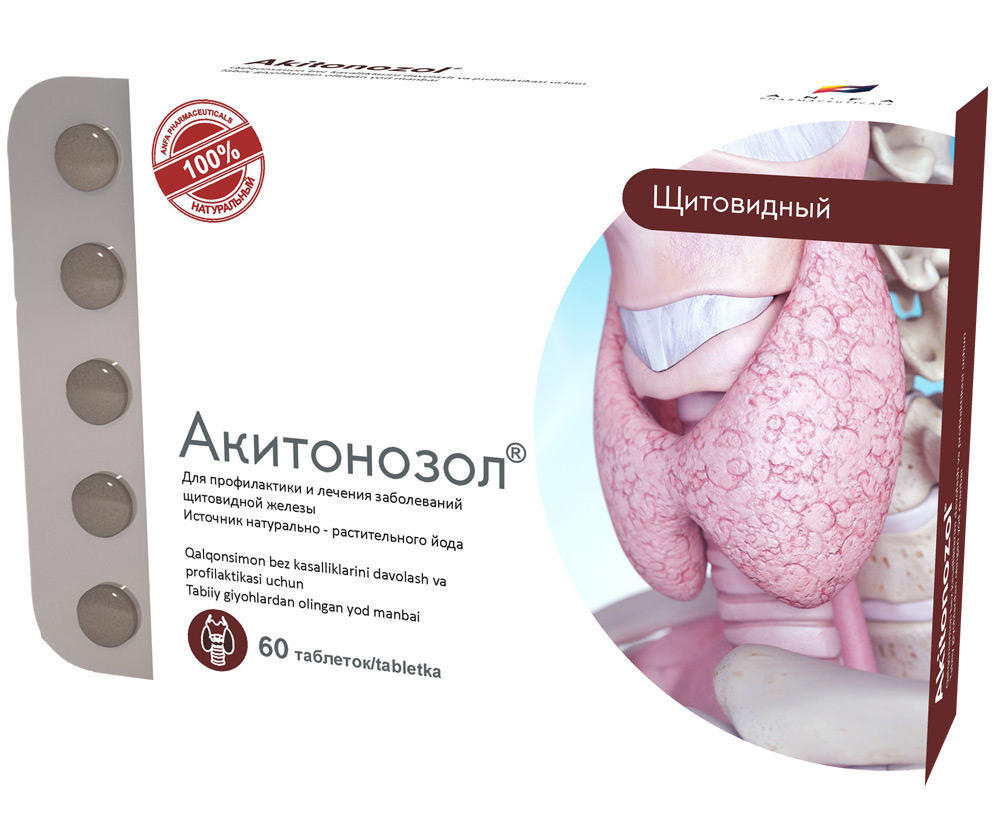
AKITONOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets made of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label, (2) – instructions for use.
Composition:
Common cocklebur (Xanthium strumarium L.),
Sweet flag (Acorus calamus L. ),
Garlic (Allium sativum L.),
Laminaria – seaweed (Laminaria J.V. Lamour L.),
Creeping Thyme (Thymus serpyllum L.),
Common Sage (Salvia officinalis L.),
Walnut (Juglans regia L.),
Perforate St John’s-wort (Hypericym perforatum L.).
EFFECT OF THE MEDICINE
AKITONOZOL is used for the prevention and treatment of thyroid diseases. The micronutrient iodine is an essential micronutrient necessary for normal operation of the thyroid gland. Iodine is not synthesized in the body, therefore, all the necessary amount should be ingested from the outside. When there is an optimum amount of iodine, development of various thyroid diseases, including endemic goiter can be prevented. The medicine compensates the lack of iodine supplied with food to the body, which is especially important in endemic areas, i.e. in areas where the soil and etc. have an insufficient amount of iodine.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Prevention of thyroid diseases caused by lack of iodine in the body, as well as for the prevention of iodine deficiency in patients in need of an increased intake of iodine (pregnant women, nursing mothers, children, and adolescents);
- Prevention of recurrence of goiter after thyroid surgery or after pharmacological treatment of goiter;
- Treatment of several diseases caused by insufficient intake of iodine: diffuse nontoxic goiter, diffuse euthyroid goiter.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
Intolerance to iodine preparations.
DOSAGE
The duration of treatment and the dose are determined by a physician for each patient individually.
CHILDREN AGED 12 YEARS OLD AND BELOW
ADULTS AND CHILDREN 12 YEARS OLD AND ABOVE
PREGNANT AND NURSING WOMEN
1 tablet once a day in the morning with a meal
2 tablets once a day in the morning with a meal
2-3 tablets once a day in the morning with a meal
The course of administration is usually from one to three months. The treatment can be repeated a month after the end of the previous course.
SIDE EFFECTS
Usually, there are no side effects. In most cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of AKITONOZOL overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.