DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic,
diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
– Biologic corrector of carbohydrate metabolism;
– Normalizes carbohydrate metabolism and lowers blood sugar levels;
– Compensates energy deficit;
– When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
– Obesity, contributes to the reduction and stabilization of weight;
– Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
SYRUP
2.5-5.0 ml 3 times a day
The medicine is administered with meals with plenty of water.
Course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
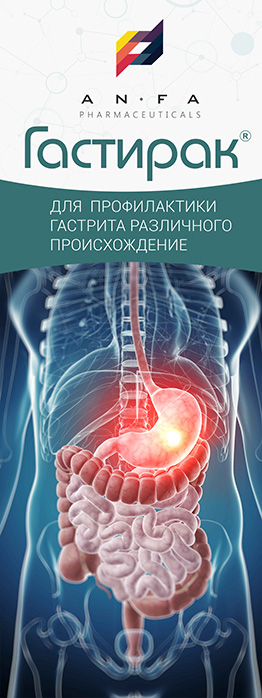
GASTIRAK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Myrtle flag (Acorus cálamus)
Plantain (Plantago L.)
Walnut (Júglans régia)
Aloe (Aloe L.)
Chamomile (Matricaria L.)
Melissa (Melissa offlcinalis L.)
EFFECT OF THE MEDICINE
GASTIRAK is a combined medicine with antacid, astringent, antispasmodic, and laxative properties.
– helps to reduce the activity of pepsin and gastric acidity.
– provides restorative, antibacterial and anti-inflammatory action, forming a protective film on the gastric mucosa.
– the active ingredients of aloe and myrtle flag are characterized by antispasmodic activity, and Melissa has a laxative effect.
INDICATIONS
- Hyperacid gastritis;
- Non-ulcer dyspepsia;
- Gastroesophageal reflux disease;
- Peptic and duodenal ulcer, including ulcer associated with Helicobacter pylori.
- Gastralgia, acid regurgitation;
- Heartburn of different etiology;
- The medicine is also used for the symptomatic treatment of pain in the stomach caused by diet non-compliance, alcohol abuse, smoking and treatment with medicines that are irritating to the gastric mucosa.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
Renal failure.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
CHILDREN BETWEEN 6 AND 12 YEARS OF AGE
1-2 tablets three times a day
1 tablet three times a day
GASTIRAK is recommended to take 30-60 minutes after the meal. Crush the tablets into powder and take with 100 ml of water.
The course of administration is usually from one to three months; treatment can be repeated a month after the end of the previous course.
SIDE EFFECTS
Usually, there are no side effects. In most cases, the medicine is well tolerated. In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of GASTIRAK overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
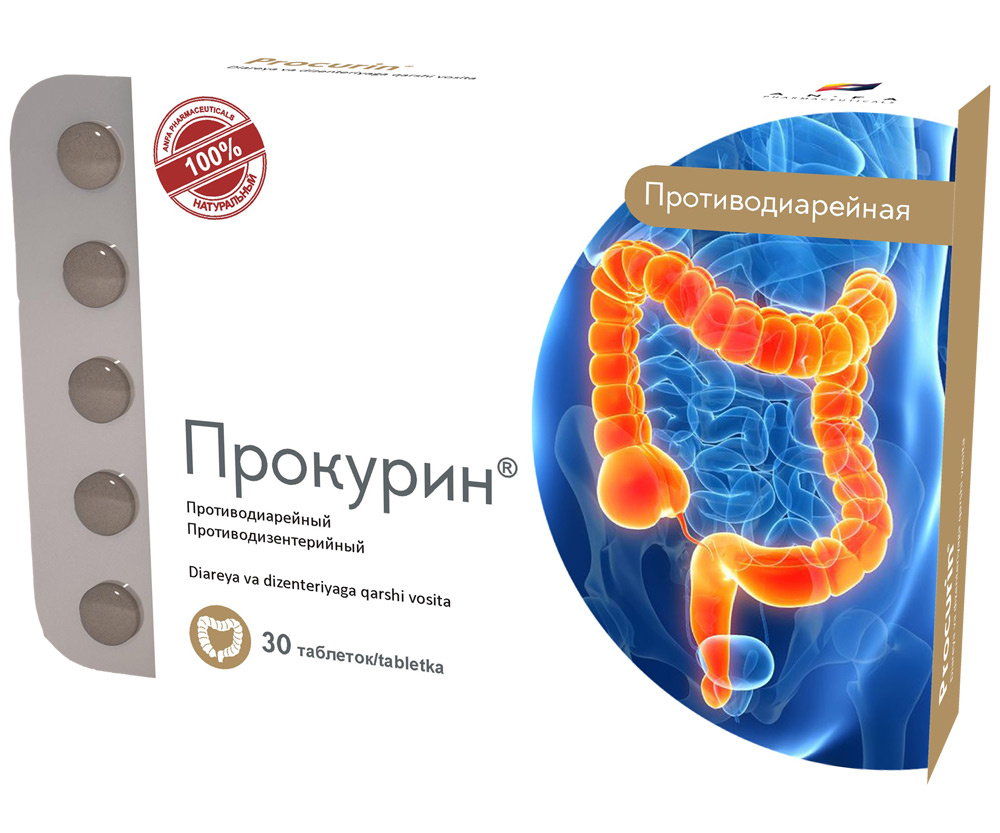
PROCURIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 30 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Common knotgrass (Polýgonum aviculáre L.)
Burnet Bloodwort (Sanguisórba officinális L.)
Elecampane Inula (Ínula helénium L.)
Perforate St John’s-wort (Hypéricum perforátum L.)
Sandy Everlasting (Helichrýsum arenárium L.)
Walnut (Júglans régia L.)
Horse Sorrel (Rúmex confértus L.)
Peppermint (Méntha piperíta L.)
EFFECT OF THE MEDICINE
- The medicine acts as a combined antidiarrheal, astringent, anti-inflammatory, disinfection action.
- It has a calming, antispasmodic, antiseptic and analgesic effect on the mucosa of the gastrointestinal tract.
- The antidiarrhoeal effect is achieved by reducing of vermicular movement, intestinal and secretory activity of intestinal tract, and accelerates the regeneration of the mucous membranes of the gastrointestinal tract.
- It has antimicrobial activity (action aimed at the destruction of germs); it normalizes the intestinal flora.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
During treatment, fatty foods, alcohol, and melons need to be proscribed from the diet.
It is necessary to follow a diet and short breaks between meals are recommended with a gradual return to the original nature of the diet, drink plenty of fluids, avoid eating spices and limit the reception of flour products.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
If necessary the treatment course can be repeated.
INDICATIONS
- non-specific acute and chronic diarrhea;
- diarrhea associated with allergic reactions;
- diarrhea caused by psycho-emotional factors;
- diarrhea due to changes in diet and eating habits;
- colitis;
- dysbacteriosis after taking broad-spectrum antibiotics (prevention and treatment);
- enteritis, accompanied by diarrhea;
- flatulence;
- the medicine is used as an aid for infectious diarrhea.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 years old and below as a therapeutic enema
CHILDREN 3 years old and above – per oral administraion
ADULTS
2 tablets for 100 ml of water
1 tablet 3 times a day 5-10 minutes before the meal
2 tablets 3 times a day 5-10 minutes before the meal
The duration of treatment depends on the reason of dysbacteriosis development and individual peculiarities of the body.
The course of treatment in chronic diseases is usually 5 – 10 days.
If necessary the treatment course can be repeated.
OVERDOSE
The cases of PROCURIN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
VETROGONIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Chamomile Extract (Matricaria chamomilla)
Creeping Thyme Extract (Thymus vulgaris L.)
Pepper Mint Extract (Mentha piperita L.)
Perforate St John’s-wort Extract (Hypéricum perforátum L.)
Elecampane Inula Extract (Inula helenium L.)
Milfoil Extract (Achillea millefolium L.)
Fennel Extract (Foeniculum vulgare Mill.)
Effect of the medicine
- The effect of the medicine arises from its components:
- Menthol in the leaves of peppermint dilates blood vessels of the heart, lungs, and brain;
- Essential oil, flavonoids, and phytoncide in the fruits of fennel and thyme;
- Chamomile is used for intestinal cramps, flatulence, and diarrhea, as well as a diaphoretic;
- Inula helps both at stomach and intestine diseases such as ulcers, gastritis, diarrhea of noninfectious nature and colitis;
- Perforate St John’s-wort contains hardening agents, essential oil, β-sitosterol, triterpene saponins, flavonoids (hyperoside, rutin), vitamins C, E, anthraquinones, macro- and micronutrients and other biologically active substances, the infusion of herbs is used as an astringent and antiseptic in the gastrointestinal diseases;
- Milfoil has anti-bacterial and anti-inflammatory properties. This herb has a positive effect on the human internal organs, as well as it promotes mucus formation, relieves gas in the intestines.
INDICATIONS
- Excessive formation and accumulation of gases in the digestive tract: symptoms of flatulence (feeling of fullness in the epigastric region, flatulence), aerophagia, dyspepsia;
- Intestinal colic in newborns and infants;
- Hyper flatulence after surgery;
- Tensides Poisoning (including surfactants, containing in the detergents) as a defoamer.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
During flatulence and colic:
NEWBORNS AND INFANTS
CHILDREN FROM 1 TO 6 YEARS OLD
CHILDREN BETWEEN 7 AND 12 YEARS OF AGE
ADULTS AND CHILDREN FROM 12 YEARS OLD AND ABOVE
0.5-1.0 ml. during nursing or before/after each feeding
1.0-2.5 ml. 3-4 times a day
2.5-5.0 ml 3-4 times a day
5.0-7.5 ml. 3-4 times a day
When poisoning with detergents the medicine is prescribed for adults in a dose of 5.0-10.0 ml. and for children in a dose of 1.0-5.0 ml. of syrup depending on the severity of poisoning.
The medicine is taken before or after meals. The course of administration is 14 days.
The course can be repeated after a weak.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
NOMAGISSAN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
One-sided-wintergreen (Orthília secúnda L.)
Oregano (Origanum vulgáre L.)
Rose Hips Fruits (Frūctus Rosāe)
Chamomile (Matricāria chamomīlla L.)
Marigold Officinalis (Caléndula officinális L.)
Shepherd’s purse (Capsélla búrsa – pastóris L.)
Sandy Everlast (Helichrýsum arenárium L.).
EFFECT OF THE MEDICINE
- Combination herbal menstrual cycle regulator.
- It promotes the elimination of post-menopausal bleeding by stimulating and strengthening the muscles of the endometrium and ovarian tissue.
- It has anti-inflammatory and antibacterial properties.
- It helps in premenstrual syndrome, as the medicine monitors and aligns hormone levels.
- It helps metabolism, restores immunity (protective properties of the body) and has a health improving properties.
SIDE EFFECTS
Usually, there are no side effects; in the majority of cases, the medicine is well tolerated. In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Dysmenorrhea, amenorrhea, hypermenorrhoea (in the complex therapy);
- Premenstrual syndrome;
- Abnormal vaginal discharge;
- Postmenopausal bleeding;
- White vaginal discharge caused by blockage of cervical glands (Leucorrhœa);
- Copious menstruation (Menorrhagia);
- Uterine bleeding not related to the menstrual cycle (Metrorrhagia).
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine, pregnancy, lactation.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
2 tablets 3 times a day 5-10 minutes before the meal.
The course of treatment is 15 days.
If necessary, the treatment course can be repeated.
OVERDOSE
The cases of NOMAGISSAN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DERMOTONAL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
| Composition: | |
| Perforate St John’s-wort Extract (Hypéricum perforátum) | |
| Common verbena Extract (Verbéna officinalis) | |
| Milfoil Extract (Achillea millefolium L.) | |
| Chamomile Extract (Matricaria) | |
| Wild pansy Extract (Víola trícolor) |
EFFECT OF THE MEDICINE
- Antimicrobial action;
- Anti-allergic action;
- Antifungal action;
- Astriction, coating and adsorbing effect;
- Tones and softens the skin.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
Dermotonal is used in the treatment and prevention of the following:
- Oily seborrhea of the face;
- Rash, scrofula (exudative diathesis, skin itch);
- Dermatitis, trophic ulcers, furunculus, abscesses (pyodermatitis, eczema);
- Phlegmonous acne;
- Keratoderma, trichophytosis;
- Endometritis, endometriosis, fibromatous bleeding;
- The diseases of the uterus, vagina, cervix (cervical erosion).
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine. Menstrual cycle.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Bath:
Infants 0-3 years old: 1 tablespoon of syrup + 0.7 l of water
Children 4-14 years old: 2 tablespoon of syrup + 0.7 l of water
Adults: solution 1: 6
Lotion:
A medicated swab is directly applied to the skin
Irrigation:
Oral cavity – 100 ml of water + 1 tablespoon of syrup
Gynecology – 200 ml of water + 1 tablespoon of syrup
The medicine is intended for external and local application.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
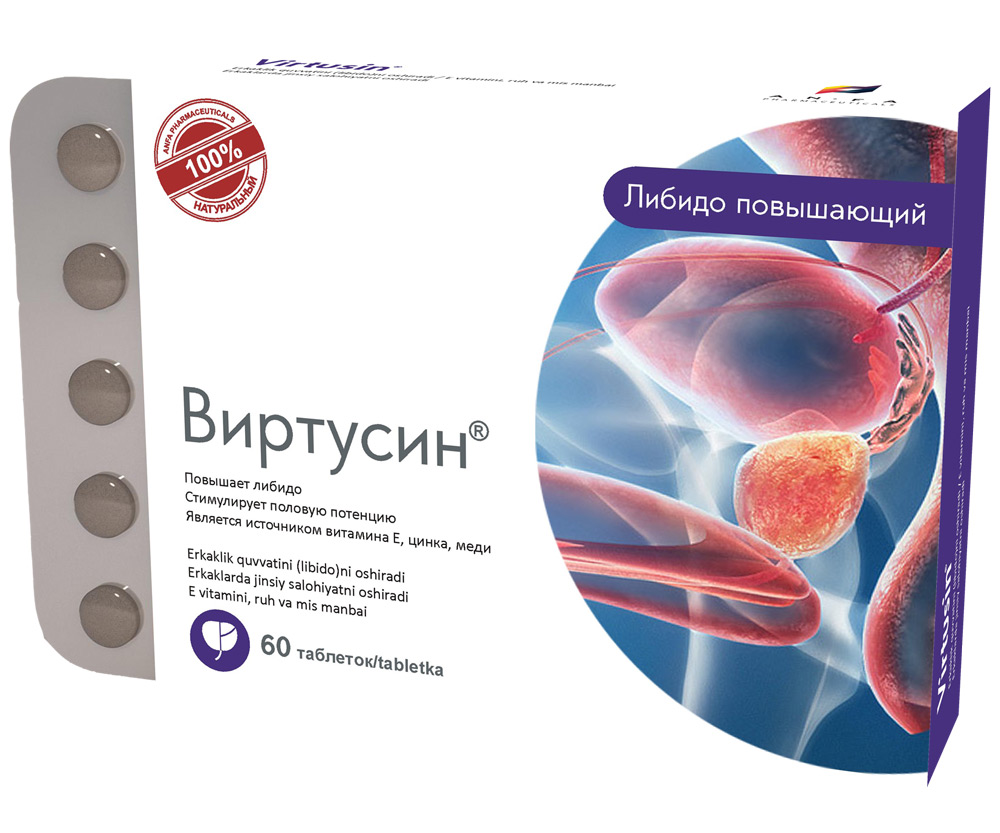
VIRTUSIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective
holographic label, (2) – instruction for use.
Composition:
Anomalous Peony (Paeonia anomala L.)
Rosebay Willow-herb (Chamaenerion angustifolium L.)
Aspen (Populus tremula L.)
Pumpkin (Cucurbita pepo L.)
Horse Chestnut (Aesculus hippocastanum L.)
Common nettle (Urtica dioica L.)
Greater Burdock (Arctium lappa L.)
EFFECT OF THE MEDICINE
- Increases libido, energy, and endurance.
- Normalizes the function of urinary organs in men.
- The medicine is a source of vitamin E, zinc, copper.
- Promotes the improvement and maintenance of the prostate gland function.
- Stimulates sexual potency in men and enhances the reproductive abilities.
INDICATIONS
- Prevention or treatment of acute prostatitis and prostatic adenoma;
- Cystitis;
- Prevention of chronic prostatitis exacerbations;
- Additional long-term therapy after suffering prostatitis;
- Functional disorders of the genitourinary system;
- Reduced erection;
- For normalization of functions and structures of the prostate and improvement of metabolism in its cells.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal
The course of treatment in chronic diseases is usually 30 days.
If necessary the treatment course can be repeated.
SIDE EFFECTS
Usually, there are no side effects. In the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of VIRTUSIN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
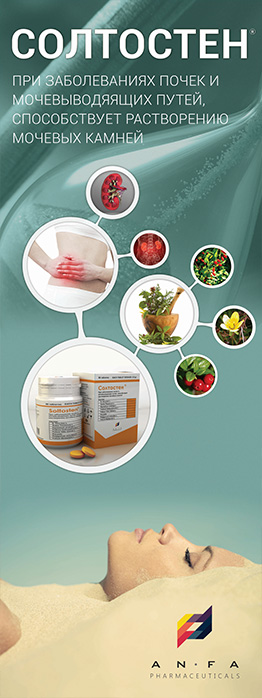
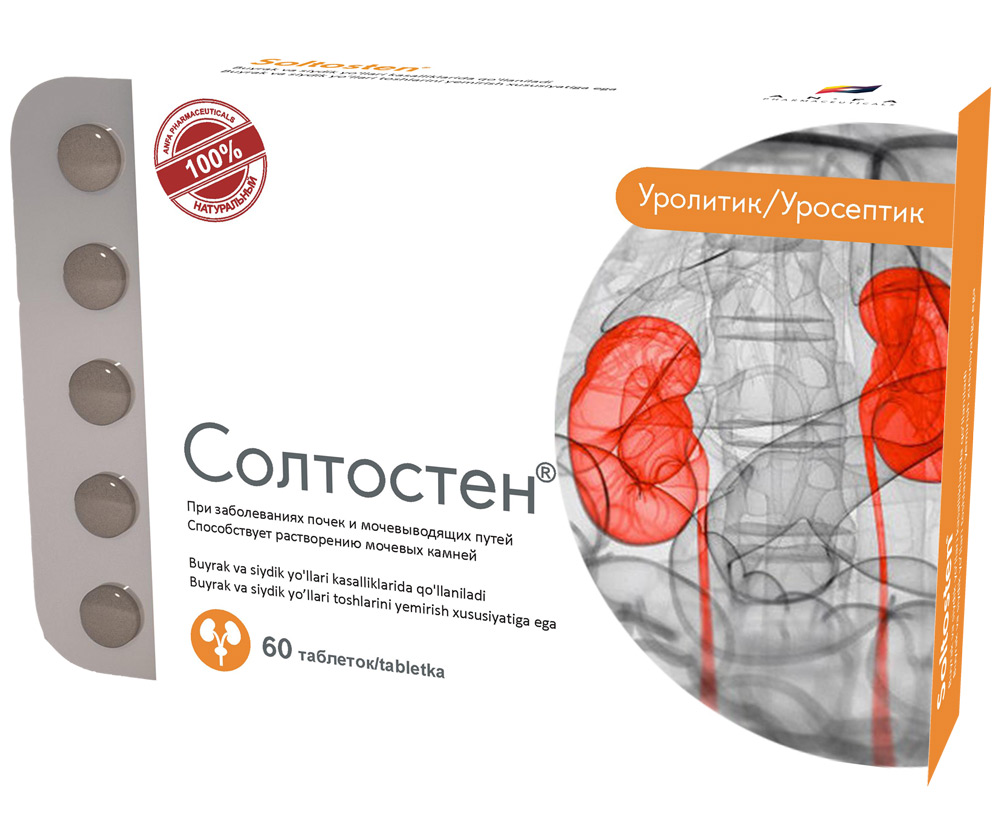
SOLTOSTEN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Yellow Marsh Saxifrage (Saxifraga hirculus L.)
Common Madder (Rubia tinctorum L.)
Common Horsetail (Equisetum arvense L..)
Indian Kidney Tea (Folium Orthosíphonis L.)
Clusterberry Leaf (Vaccinium vitis-idaea L.)
Mountain Knotgrass (Aerva lanata)
Prostrate Knot Grass (Polygonum aviculare L.)
Common Barberry (Berberis vulgaris L.)
EFFECT OF THE MEDICINE
- The medicine has antispasmodic, diuretic and anti-inflammatory effect.
- Promotes the discharge of stones of various nature in nephrolithiasis, normalizes salt metabolism.
SIDE EFFECTS
Usually, there are no side effects, in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Chronic inflammatory diseases of the kidney and urinary tract (pyelonephritis, pyelitis, cystitis, urethritis, prostatitis).
- Urolithiasis.
- Cholelithiasis, cholecystitis, cholangitis.
- Urine acid diathesis.
- Osteochondrosis, gout.
- Moreover, the medicine is prescribed in the postoperative period after removal of kidney stones or their spontaneous discharge.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 YEARS OF AGE AND ABOVE
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal.
2 tablets 3 times a day 5-10 minutes before the meal.
The course of treatment in chronic diseases is usually 20 – 30 days.
If necessary the treatment course can be repeated.
OVERDOSE
The cases of SOLTOSTEN overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
SILOPIK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
| Bindii Extract (Tríbulus terréstris) | |
| Liquorice Extract (Glycyrrhí́za glábra) |
EFFECT OF THE MEDICINE
Silopik – a preparation of plant origin.
- Possesses antimicrobial, anti-sclerotic, anti-inflammatory, vasodilating, anticoagulant action;
- It also regulates the water-salt balance and has a stimulating effect on the male sex glands;
- Silopik is a hypocholesterolemic agent. Its use inhibits the absorption of cholesterol in the intestine, reduces blood clotting, increases the tone of the small intestine and stimulates its contraction;
- Stimulates potency. It is considered as a strengthening tonic;
- Silopik is taken with constipation, for stimulation of bile secretion, as a diuretic;
- Silopik is able to cope with migraines, insomnia, colds and even noise in the ears and gonorrhea;
- Silopik with the external application is considered effective wound-healing agent, is able to cope even with burns, used for conjunctivitis, and helps to get rid of acne, fungus, inflammation.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
Instruction
In the treatment of sexual dysfunction:
- increase the level of testosterone (total and free);
- improves sexual health, libido, and erections;
- improves various parameters of male sperm, such as mobility, curvilinear velocity, vitality;
- increases sperm quality;
- promotes the treatment of sexual dysfunction in women (and during menopause);
- it is recommended to take with cystitis, pyelonephritis, urethritis, other inflammations of the genitourinary system.
While lowering the cholesterol:
- the level of cholesterol is reduced mainly by lowering LDL (low-density lipoproteins), without having a significant effect on HDL (high-density lipoproteins).
While lowering the blood pressure:
- promotes a decrease in diastolic blood pressure;
- helps reduce a headache, dizziness, insomnia, decreased heart rate.
In the treatment of IHD (Ischemic heart disease):
- dilutes blood and improves ECG results.
While lowering the blood sugar level:
- the medicine showed high efficiency in reducing glucose levels in patients with type 2 diabetes. The mechanism of sugar reduction is associated with inhibition of a-glucosidase activity in the small intestine;
- reduces glycation of proteins;
- possesses a slight alpha-amylase-inhibiting effect that is dose-independent, which indicates the effectiveness of an application with very small dosages;
- possesses lipase inhibitory properties that are useful for weight loss, fat loss in diabetic patients.
As a diuretic effect:
- the study showed an increase in diuresis during the day by 200 ml.
When used in sports:
- reduces the percentage of fat in the body, significantly increases muscle mass, as well as an increase in the level of dihydrotestosterone;
- facilitates an easier transfer of heavy physical, intellectual and mental stress, increases the body’s resistance to severe illnesses.
CONTRAINDICATION
- pregnancy;
- people under the age of 16;
- neurogenic and endocrine constipation;
- acute stomach syndrome;
- appendicitis;
- adenoma of the prostate gland.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
With a decrease in libido, impotence, and infertility. Inside, after a meal.
Men with reduced libido, impotence, and infertility, a dosage of 10-15 ml 3 times a day is recommended. The duration of treatment is at least 90 days. The course of treatment can be repeated until a positive therapeutic effect is obtained.
Women with endocrine infertility, a dosage of 5-10 ml are recommended 3 times a day. From the 1st to the 12th day of the menstrual cycle.
This course can be repeated periodically until the onset of pregnancy.
Dislipoproteinemia. 10 ml 3 times a day. Duration of treatment – at least 3 months.
Women with climacteric and post-stroke syndrome, 5-10 ml tablets 3 times a day for 60-90 days.
After the improvement of the condition, gradually lower to the dose – 5 ml per day for 1-2 years.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
ANEMAK
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Common Nettle Extract (Urtica dioica L.)
Eleutherococcus Extract (Eleutherococcus senticosus)
Milfoil Extract (Achillea millefolium L.)
Aloe Extract (Aloe arborescens Mill.)
Trifid Bur-marigold Extract (Bidens Tripartita)
Excipients: purified water up to 100 ml, Sugar, Ethyl alcohol (95%)
EFFECT OF THE MEDICINE
– When using the medicine has a gradual regression effect on clinical and laboratory symptoms of anemia;
– Restores iron deficiency in the body and stimulates the synthesis of hemoglobin in iron deficiency anemia;
– Increases iron absorption in the intestine.
– It is a source of iron and essential amino acids;
– It helps overcome fatigue, depression, frequent tiredness, dizziness, increases the body defenses;
INDICATIONS
– Anemia (iron deficiency of different degrees of severity);
– Vitamin B12 deficiency anemia;
– Pregnancy and lactation (prevention of iron deficiency during pregnancy and lactation);
– Retardation of physical and mental development, and intensive growth;
– Sports and physical exercise, intense physical labor;
– Poor nutrition, unbalanced diet;
– Frequent acute respiratory viral infections and acute respiratory diseases (in the complex therapy);
– Donation of blood;
– For patients after various surgical measures;
– At prolonged bleeding;
– For children in adolescence and for adults (e.g., vegetarians and the elderly);
– Prevention of iron deficiency anemia in patients of a risk-group in the case where there is no possibility to ensure adequate intake of iron from food.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Anemak is recommended to take:
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
CHILDREN BETWEEN 3 AND 12 YEARS OF AGE
5 – 10 ml., 3 times a day with a meal
2,5 – 5 ml., 2-3 times a day with a meal
For peroral use, 5-10 minutes after the meal.
The course of administration is usually 30 days.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation. The use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.