DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label,
(2) – instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic, diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Biologic corrector of carbohydrate metabolism;
- Normalizes carbohydrate metabolism and lowers blood sugar levels;
- Compensates energy deficit;
- When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
- In Obesity, medicine contributes to the reduction and stabilization of weight;
- Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
INSIDE
2 tablets 3 times a day
The medicine is administered with meals with plenty of water.
The course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
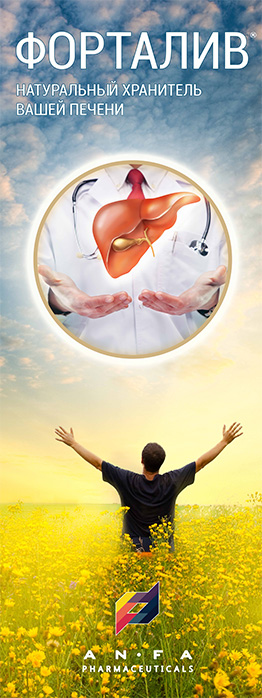
FORTALIV
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
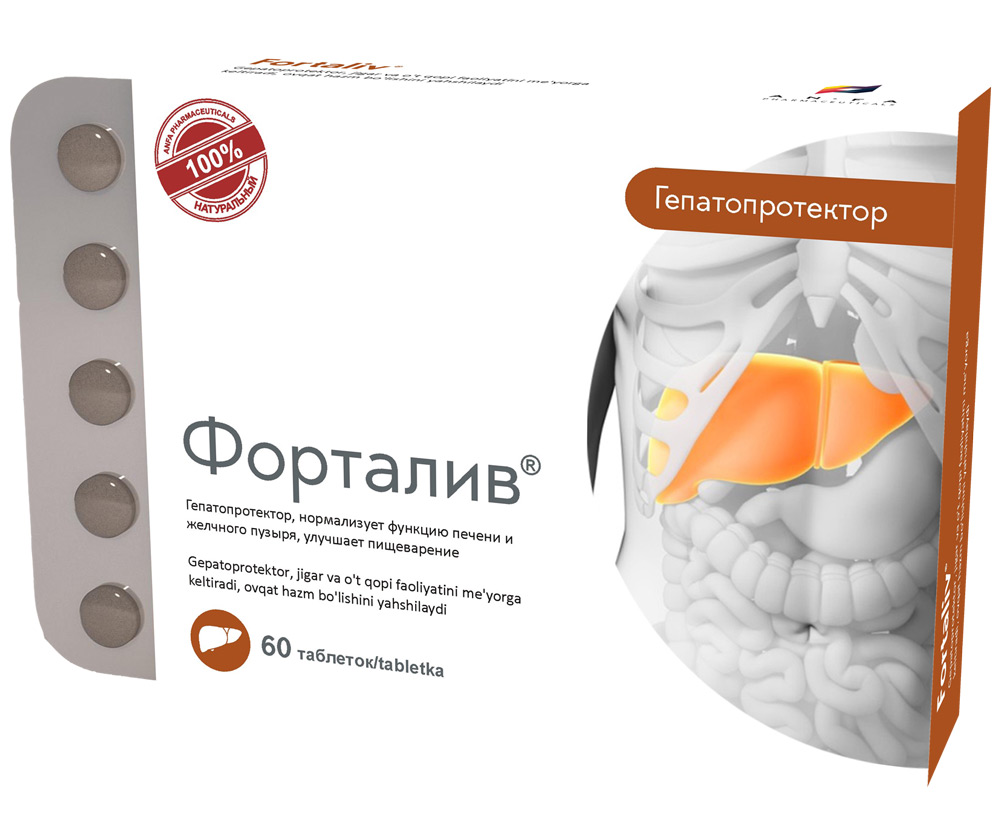
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Sandy Everlasting (Helichrýsum arenárium L)
Common Agrimony (Agrimónia eupatória L.)
Corn Silk (Stigmata Maydis L.)
Tansy (Tanacétum vulgáre L.)
Milk Thistle (Sílybum mariánum L.)
EFFECT OF THE MEDICINE
- Combination of herbal hepatoprotector;
- Promotes the regeneration of liver cells and stimulates their biosynthetic function;
- Reduces the impact of toxins on liver cells and eliminates biliary dyskinesia symptoms;
- Improves appetite and normalizes digestion;
- Possesses choleretic, anti-oxidant, anti-inflammatory and a slight diuretic effect.
SIDE EFFECTS
Usually, there are no side effects, in the majority of cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions, dyspeptic disorders of the gastrointestinal tract.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATIONs
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine is prescribed for:
- chronic and acute (during re-convalescence);
- hepatitis of various etiologies (viral, drugs, alcohol, etc.);
- Cirrhosis of the liver and pre-cirrhosis states (in the complex therapy);
- Fatty liver Disease and biliary dyskinesia;
- Cholecystitis.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN 3 YEARS OF AGE AND ABOVE
ADULTS
1 tablet 2 times a day 5-10 minutes before the meal.
2 tablets 3 times a day 5-10 minutes before the meal.
The course of treatment in chronic diseases is usually 20 – 30 days. If necessary the treatment course can be repeated.
OVERDOSE
The cases of FORTALIV overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicineshould be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 2 years
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DEPARAZITOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) spoon – dispenser; (3) instruction for use.
Composition:
Tansy Extract (Tanacetum vulgare L.)
Chamomile Extract (Matricaria Chamomilla L.)
Perforate St John’s-wort Extract (Hypericum L.)
Yarrow Extract (Achillea millefolium L.)
Dwarf everlast (Helichrysum Arenarium L.)
Pumpkin Seed Extract (Cucurbita pepo L)
EFFECT OF THE MEDICINE
DEPARAZITOL — Anthelmintic preparation of a wide spectrum of action. It is most effective against Enterobius vermicularis, Trichuris trichiura, Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus, Strongyloides stercoralis, Taenia solium, Echinococcus granulosus, Echinococcus multilocularis, Trichinella spiralis, Trichinella nativa, Trichinella nelsoni.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation. Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine Deparazitol is prescribed for:
- enterobiasis;
- ascaridosis;
- ancylostomiasis;
- strongyloidiasis;
- trichocephalus;
- trichinosis;
- taeniasis;
- mixed helminthiases.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Deparazitol is recommended to take: Inside with a little amount of water.
CHILDREN OVER 3 YEARS OF AGE:
– when enterobiasis – 5 ml. 1 time per day,
– with mixed helminthiases – in the morning and in the evening 10ml. for 7 days.
If the symptoms persist, it is recommended to repeat the course of treatment after 3 weeks.
ADULTS AND CHILDREN OVER 14 YEARS OF AGE:
– when trichinellosis – on the first day for 10-15 ml. 3 times a day, on the second day for 10-15 ml. 4 times a day, and from 3 to 14 day – 25 ml. 3 times a day.
– with ascariasis, trichocephalosis, ankylostomiasis, teniosis, strongyloidiasis, and mixed helminthiases – in the morning and in the evening 10 ml. for 3 days.
Duration of intake – from one to three months, the course of therapy can be repeated one month after the end of the previous course.
It is recommended that all members of the family be treated at the same time.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
GINKO CARDIOZEN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Ginkgo biloba (Ginkgo L.)
Elecampane (Inula helenium L.)
Midland hawthorn (Crataegus laevigata)
Valerian herb (Valeriana officinalis)
Lemon balm (Melissa officinalis)
Common yarrow (Achillea millefolium L)
Motherwort (Leonúrus)
Peppermint (Mentha piperita L.)
effect of the medicine
- GINKO CARDIOZEN – a preparation of herbal origin. The medicine has a spasmolytic effect of myotropic nature (similar to the effect of papaverine), mainly in relation to coronary vessels.
- 3-5 days after the intake of Ginko Cardiozen, the therapeutic effect of the medicine occurs – attenuation is observed or elimination of the manifestations of diseases (elimination or significant attenuation of chest pains, pain in the heart, etc.).
- Treatment with Ginko Cardiozen significantly reduces and weakens the attacks of angina, patients less often resort to the use of nitroglycerin, Validol, and other vasodilators.
- Contributes to the normalization of the heart rate. The medicine contributes to the normalization of the contractile function of the myocardium, improving conductivity, and provides nutrition to the heart muscle.
- The main components of the medicine (hawthorn and motherwort) affect all the basic mechanisms that optimize the work of the heart, improves the nutrition of the heart muscle, by dilating the coronary vessels, improves metabolic processes in the myocardium at the cellular level, regulates the heart rate.
- Moreover, possesses a sedative effect, which is important in the prevention of heart diseases.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicine GINKO CARDIOZEN is prescribed for:
- heart failure;
- cardioneurosis;
- cardio-psychoneurosis;
- arterial hypertension;
- cardiac ischemia;
- chronic coronary insufficiency; (manifested by pain behind the sternum and in the region of the heart at rest or after physical exertion).
- myocardial dystrophy of different genesis;
- disorders of cerebral circulation;
CONTRAINDICATIONS
- diagnosed gastritis of erosive type;
- stomach ulcer;
- peptic ulcer of the duodenum in the stage of exacerbation;
- acute myocardial infarction;
- stable low blood pressure – (Arterial hypotension);
- disorders of cerebral circulation in an acute phase.
- hypersensitivity to one or more components of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually. GINKO CARDIOSEN is recommended to take:
Inside
1 tablet 2-3 times a day, for half an hour before meals.
The duration of treatment with the medicine (from 2 to 4 weeks) is determined by the patient’s individual response to treatment.
Repeated use of the medicine is recommended after the interval of 5-10 days. The medicine can be used not only in inpatient but also in outpatient basis.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
TROMBORON
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets of herbs, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with protective holographic label, (2) – instruction for use.
Composition:
Ginkgo biloba (Ginkgo biloba L.)
Feverfew (Tanacetum parthenium L..)
Garlic (Allium sativum L.)
Ginger (Zingiber officinale)
Garden angelica (Angelica archangelica officinalis)
EFFECT OF THE MEDICINE
- Tromboronis designed for the purification of vessels from excess cholesterol in hyperlipidemia IIa type.
- Tromboron significantly reduces the level of total cholesterol, reduces the concentration “bad” cholesterol and improves the concentration of “good” cholesterol that is vital for the proper functioning of the brain and the production of hormones such as testosterone and estrogen.
- Tromboronis used as a prophylaxis for the protection of blood vessels of the heart and brain from cholesterol.
- It is recommended to begin prophylaxis to all above35-40 years.
- With regular admission, Tromboron removes cholesterol from the body and prevents its deposition on the walls of the vessels in the form of atherosclerotic plaques.
- Moreover, Troboron normalizes the permeability of the vascular wall.
SIDE EFFECTS
Usually, side effects do not occur, in most cases, the medication is well tolerated.
There may be allergic reactions (itching, rash), as well as nausea, headache, a feeling of bitterness in the mouth.
Use of the medicine should be discontinued when hypersensitivity reactions occur and consult a doctor.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
The medicineTromboron is prescribed for:
- hyperlipidemia type IIa according to Frederickson, weakly expressed;
- prophylaxis of acute myocardial infarction in the presence of risk factors (such as diabetes mellitus, hyperlipidemia, arterial hypertension, obesity, smoking, old age);
- secondary prophylaxis of myocardial infarction;
- unstable angina;
- prevention of stroke (including in patients with transient disorders of cerebral circulation);
- prevention of thromboembolism after surgery and invasive vascular interventions (such as coronary artery bypass grafting, carotid endarterectomy, arteriovenous shunting, carotid angioplasty);
- prevention of deep vein thrombosis and thromboembolism of the pulmonary artery and its branches (for example, with prolonged immobilization as a result of extensive surgical intervention).
CONTRAINDICATIONS
During pregnancy and lactation.
Hypersensitivity to some components of the medicine, under the age of 18, and in cases of brain injury, brain diseases, liver disease, severe renal dysfunction, alcoholism.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually. Tromboron is recommended for:
INSIDE
2 tablets, 2-3 times a day for half an hour before meals.
The duration of treatment with the medicine (from 3 to 6 months) is determined by the patient’s individual response to treatment.
Conducting a second course of treatment is possible on the recommendation of a doctor.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
GORLOZIN
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Liquorice Extract (Glycyrrhiza glabra)
Ginger Extract (Zingiber officinale)
Marigold Extract (Calendula officinalis)
Turmeric Extract (Curcuma longa)
Chamomile Extract (Matricaria chamomilla)
Aloe Extract (Aloe arborescens)
Piper Cubeba Extract (Piper cubeba L.)
EFFECT OF THE MEDICINE
– Shows fungicidal and virostatic action;
– Has anti-inflammatory, antiseptic and antibacterial effect;
– Increases immunity;
– Liquorice and Calendula reduce pain, promotes relaxation, relieves inflammation, covers, contributes to the removal of phlegm and gives an antiseptic effect;
– Chamomile relieves inflammation of the throat and tonsils;
– Turmeric relieves inflammation, destroys the germs;
– Ginger Officinale relieves inflammation;
– Piper Cubeba relieves inflammation, promotes tissue restoration;
– Aloe relieves inflammation and helps to restore tissue.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation. Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial. Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
– Comprehensive Elimination of chronic and acute tonsillitis (for the oral mucosa treatment in patients with infectious and inflammatory diseases of the mouth);
– Acute laryngitis;
– Unspecified Acute tonsillitis (agranulocytic angina);
– Acute upper respiratory infections of multiple and unspecified sites;
– Chronic, atrophic pharyngitis
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
Per os::
CHILDREN BETWEEN 1 AND 6 YEARS OF AGE
CHILDREN BETWEEN 7 AND 12 YEARS OF AGE
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
1.0-2.0 ml 3 times a day
2.0-2.5 ml 3 times a day
2.5-5.0 ml 3 times a day
The medicine should be taken at least 15 days to achieve a therapeutic effect.
External use: Gargle at: Laryngitis and Tonsillitis: gargle with the solution (diluted with purified water 1: 6) 6-10 times a day
HOW TO GARGLE:
– When gargle it is necessary to tilt the head back and stick out the tongue forward as much as possible so the solution can pass into the throat as deeply as possible.
– Gargling solution should be warm, as cold water will only worsen the condition, and hot water can cause burns and unnecessary pain and stress for the patient.
– Make the sound “ahhh” to improve tonsils irrigation and prevent interference of the tongue root with the passage of the solution.
– An important condition for proper gargling is the time of the procedure, at least 30 seconds is necessary for each gargling so that the liquid could thoroughly rinse the throat.
– In order not to swallow the solution, it is important to control the breathing.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DENTOVITAL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Common Sage Extract (Salvia officinalis L.)
Licorice Extract (Glycyrrhiza glabra L.)
Milfoil Extract (Achillea millefolium L.)
Chamomile Extract (Matricaria)
Marigold Extract (Calendula officinalis)
EFFECT OF THE MEDICINE
– Antimicrobial and anti-inflammatory action;
– Astriction and antiseptic action;
– Local anesthetic.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
indications
Dentovital is used in the treatment and prevention of infectious and inflammatory diseases of the oral cavity:
– Stomatitis: acute, ulcerative, bacterial, ulcerous-erosive;
– Periodontitis: slight, moderate, severe;
– Gingivitis: acute, chronic, necrotizing ulcerative, atrophic;
– Flux (odontogenic periostitis). Scurvy (in the complex therapy);
– Hygiene treatment of removable dentures;
– To eliminate bad breath.
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
For mouthwash, dilute 10-15 ml of the medicine with 100 g of water at a room temperature.
In acute diseases, the dose can be increased up to 30 ml.
It is recommended to rinse the mouth 3-4 times a day or after every meal.
The course of administration is 7 days.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
KASHLINET
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; – (2) Spoon – dispenser; (3) instruction for use.
Composition:
Athaea Extract (Althaea officinalis)
Camel’s-thorn Extract (Alhagi)
Chamomile Extract (Matricaria chamomilla)
Licorice Extract (Glycyrrhiza glabra)
EFFECT OF THE MEDICINE
– Expectorant;
– Anti-inflammatory;
– Effect on the mucous membranes of the respiratory tract, thins mucus and reduces its viscosity.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the vial.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
KASHLINET is indicated for acute and chronic inflammatory diseases which are accompanied by difficult coughing with phlegm:
– Tracheitis;
– Bronchitis;
– Pneumonia;
– Bronchiectasis;
– Pulmonary emphysema (in adults and children aged 1-year-old and above, in such cases KASHLINET helps to improve and facilitate the expectoration of sputum).
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
For peroral use.
The duration of treatment and the dose are determined by the attending physician for each patient individually.
CHILDREN BETWEEN 1 AND 6 YEARS OF AGE
CHILDREN BETWEEN 7 AND 12 YEARS OF AGE
ADULTS AND CHILDREN ABOVE 12 YEARS OF AGE
2.5 ml (1/2 teaspoon) 3 times a day
2.5-5.0 ml (1/2-1 teaspoon) 3 times a day
5.0-7.5 ml (2-3 teaspoon) 3 times a day
The medicine is administered regardless of meals.
The course of administration is usually from 3 to 7 days.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
AKITONOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND DOSAGE FORM
Round tablets made of herbs and their fruits, with individual odor, biconvex, light – brown, speckled tablets. 60 pieces 0.5 g each. Polyethylene vials with a protective seal or packed in blisters. (1) – cardboard pack with a protective holographic label, (2) – instructions for use.
Composition:
Common cocklebur (Xanthium strumarium L.),
Sweet flag (Acorus calamus L. ),
Garlic (Allium sativum L.),
Laminaria – seaweed (Laminaria J.V. Lamour L.),
Creeping Thyme (Thymus serpyllum L.),
Common Sage (Salvia officinalis L.),
Walnut (Juglans regia L.),
Perforate St John’s-wort (Hypericym perforatum L.).
EFFECT OF THE MEDICINE
AKITONOZOL is used for the prevention and treatment of thyroid diseases. The micronutrient iodine is an essential micronutrient necessary for normal operation of the thyroid gland. Iodine is not synthesized in the body, therefore, all the necessary amount should be ingested from the outside. When there is an optimum amount of iodine, development of various thyroid diseases, including endemic goiter can be prevented. The medicine compensates the lack of iodine supplied with food to the body, which is especially important in endemic areas, i.e. in areas where the soil and etc. have an insufficient amount of iodine.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
- Prevention of thyroid diseases caused by lack of iodine in the body, as well as for the prevention of iodine deficiency in patients in need of an increased intake of iodine (pregnant women, nursing mothers, children, and adolescents);
- Prevention of recurrence of goiter after thyroid surgery or after pharmacological treatment of goiter;
- Treatment of several diseases caused by insufficient intake of iodine: diffuse nontoxic goiter, diffuse euthyroid goiter.
CONTRAINDICATIONS
Hypersensitivity to one or more components of the medicine.
Intolerance to iodine preparations.
DOSAGE
The duration of treatment and the dose are determined by a physician for each patient individually.
CHILDREN AGED 12 YEARS OLD AND BELOW
ADULTS AND CHILDREN 12 YEARS OLD AND ABOVE
PREGNANT AND NURSING WOMEN
1 tablet once a day in the morning with a meal
2 tablets once a day in the morning with a meal
2-3 tablets once a day in the morning with a meal
The course of administration is usually from one to three months. The treatment can be repeated a month after the end of the previous course.
SIDE EFFECTS
Usually, there are no side effects. In most cases, the medicine is well tolerated.
In the case of individual sensitivity to one or more components of the medicine, there may be allergic reactions.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
OVERDOSE
The cases of AKITONOZOL overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light at a temperature from + 2° C to + 25°C.
Expiration date: 24 months
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.
DIABETOZOL
- Without prescription
- Download instruction
- Share this:
- ABOUT PRODUCT
- INSTRUCTIONS
COMPOSITION AND PACKING
Syrup of herbal extracts, brown viscous liquid with individual odor (may have light sediment and clouding) in the original vials of 150 ml with a protective seal – (1) cardboard packs with protective branded holographic label; (2) Spoon – dispenser; (3) instruction for use.
Composition:
Purple Medic (Alfafa) Extract (Medicago sativa)
Jerusalem Artichoke Root Extract (Helianthus tuberosus)
Greater burdock Extract (Arctium Lappa)
Fenugreek Seeds Extract (Trigonella foenum-graecum)
Common Nettle Extract (Urtica dioica)
Lingonberry leaves Extract (Vaccinium vitis-idaea)
EFFECT OF THE MEDICINE
- Purple Medic (Alfafa) is used in malfunction of the thyroid gland and pancreas. It affects the body effectively in cases of diabetes and lowers the blood sugar level. Purple Medic removes toxins from the body. The herb has antibacterial, immune modulating and wound-healing effect, which is especially important in the development of complications such as a diabetic foot. The alkaloids contained in Purple Medic lower blood sugar level and the fibers possess absorbent properties.
- Jerusalem Artichoke Root is a source of flavonoids, and also contains inulin – a natural polymer of fructose and other bioactive substances (hemicelluloses, proteins, carbohydrates, minerals, vitamins, and carotene). The complex of active ingredients of Jerusalem Artichoke Root has a regulating effect on
carbohydrate and lipid metabolism, contributes to the normalization of intestinal flora and digestive processes. It is proved that the enrichment of diabetic patients’ diet with Jerusalem Artichoke Root helps to reduce blood
glucose levels. Jerusalem Artichoke Root is used for the prevention and treatment of diabetes type 1 and 2, atherosclerosis and pathologies in the gastrointestinal tract. - Greater burdock significantly stimulates the enzymatic activity of the pancreas. Inulin contained in abundance in the plant normalizes the number of leukocytes in blood, considerably improves metabolism.
- Research has shown that Fenugreek can lower blood sugar level by inhibiting, digesting and absorption of carbohydrates and increasing peripheral action of insulin.
- Elements of Common Nettle contribute to a gradual reduction of blood glucose levels.
- Lingonberry effectively reduces blood sugar levels. It has antimicrobial, anti-inflammatory, antipyretic,
diuretic, and choleretic properties.
SIDE EFFECTS
There may be rarely allergic hypersensitivity to separate ingredients of the preparation.
Use of the medicine should be discontinued when hypersensitivity reactions occur.
SPECIAL INSTRUCTIONS
Before use, carefully shake the syrup.
Keep the vial in a cool place after opening it. Do not heat and do not freeze.
INTERACTION WITH OTHER MEDICATION
When prescribing with other medication adverse effects were not revealed.
INDICATIONS
– Biologic corrector of carbohydrate metabolism;
– Normalizes carbohydrate metabolism and lowers blood sugar levels;
– Compensates energy deficit;
– When correcting lipid metabolism lowers cholesterol and lipids content in the blood;
– Obesity, contributes to the reduction and stabilization of weight;
– Improves peripheral blood circulation, strengthens blood vessels;
CONTRAINDICATIONS
Individual intolerance to certain ingredients of the medicine.
DOSAGE
The duration of treatment and the dose are determined by the attending physician for each patient individually.
SYRUP
2.5-5.0 ml 3 times a day
The medicine is administered with meals with plenty of water.
Course of administration is 1 month. Courses can be repeated in a week.
OVERDOSE
The cases of overdose were not reported.
STORAGE CONDITIONS AND EXPIRATION DATE
The medicine should be stored out of reach of children, protected from light, at a temperature from + 2° C to + 25°C.
Expiration date: 24 months.
The information presented on the site should not be used for self-diagnosis and treatment and can not serve as a substitute for a doctor's internal consultation.